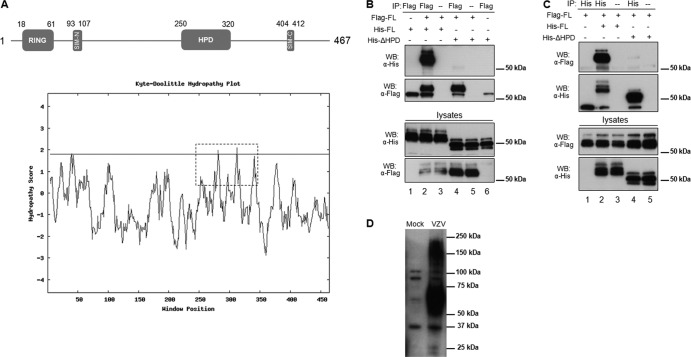
Fig 1.
Identification of a hydrophobic domain (HPD) in ORF61 and its contribution to ORF61 self-interaction. (A) Schematic representation of the ORF61 motifs (SIM-N and SIM-C) and subdomains (RING finger and HPD). The numbers represent the amino acid positions of the motifs and domains. A hydrophobicity plot of the whole ORF61 was calculated by the Kyte-Doolittle method (14), and the HPD domain is highlighted with a dashed box. (B) Coimmunoprecipitation of Flag-tagged ORF61 with His-tagged ORF61 or the His-tagged ΔHPD mutant protein. Cells were transfected with ORF61 and the ΔHPD mutant as illustrated. The transfected cell lysate was subjected to immunoprecipitation with Flag monoclonal antibody and then immunoblotted with His polyclonal antibody. (C) Reciprocal coimmunoprecipitation. The transfected cell lysate was subjected to immunoprecipitation with His polyclonal antibody and then immunoblotted with Flag monoclonal antibody. (D) ORF61 oligomerization in VZV-infected cells. VZV-infected cell lysate was subjected to SDS-PAGE and then immunoblotted with ORF61 polyclonal antibody.