Fig 1.
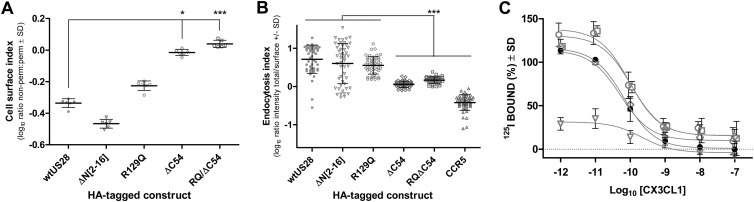
(A) Expression levels of HA-tagged US28 constructs detected by ELISA. COS-7 cells in 6-well dishes were transfected with 4 μg of the indicated HA-tagged US28 constructs or the pcDNA3 vector control using Lipofectamine 2000 (Invitrogen, Australia). At 6 h posttransfection, cells were trypsinized and seeded into replicate 96-well trays (six wells per construct per plate). The detection of the HA-tagged constructs in cells permeabilized with Triton X-100 and nonpermeabilized replicate cells was performed 24 h later as described previously (19). Receptor levels were measured as optical density (OD) readings at 450 nm and corrected for background via subtraction of mean control values (perm. or non-perm.). Data are presented as a cell surface index, i.e., the log10 ratio of the cell surface (nonpermeabilized) to total (permeabilized) OD values. Bars indicate means and standard deviations (SD) (n = 6). Asterisks indicate significant differences between the wt and mutated US28 constructs (Kruskal-Wallis with Dunn's posttest, *, P < 0.05; ***, P < 0.001). (B) Quantitative assessment of endocytosis for wt US28 and US28 mutants. HeLa cells transfected with 4 μg of the indicated HA-tagged constructs using Lipofectamine 2000 were labeled with rabbit anti-HA (ab9110, 1:500; Abcam, Cambridge, United Kingdom) at 37°C for 1 h and then “chased” in the absence of antibody for a further 20 min. Endocytosis was stopped by incubating cells at 4°C and cell surface-retained receptors labeled with Alexa Fluor 594 (AF594) goat anti-rabbit IgG (1:1,000; A11037; Molecular Probes, Invitrogen, Australia) for 1 h at 4°C. The cells were fixed with 3% paraformaldehyde, permeabilized with 0.2% Triton X-100, and incubated with AF488 goat anti-rabbit IgG (A11034, 1:1,000; Molecular Probes, Invitrogen, Australia) for 1 h at room temperature. Images from random fields captured at a magnification of ×40 were converted to grayscale; the AF488 and AF594 channels were overlaid, and the ratio of AF488 (total) to AF594 (surface) staining of individual cells was determined using NIH ImageJ (http://rsb.info.nih.gov/ij/). The log10 ratio provides an endocytosis index; this ratio increases as the proportion of endocytosed receptors increases. Data are plotted for individual cells, with bars indicating means and SD (50 cells). Asterisks indicate significant differences between the wt and other constructs (Kruskal-Wallis with Dunn's posttest, ***, P < 0.001). (C) Surface expression of wt US28 and US28 mutants determined by whole-cell binding of the chemokine ligand 125I-CX3CL1 (fractalkine) as described previously (27). Whole-cell homologous competition binding was performed in MEF infected (MOI = 3) with either wt US28 (filled circles), US28ΔN[2-16] (open triangles), US28R129Q (open circles), US28ΔC54 (open diamonds), or US28RQ/ΔC54 (open squares). At 24 h p.i., cells were labeled with 125I-CX3CL1 (fractalkine) and incubated with increasing amounts of cold CX3CL1 as indicated. Data are means ± SD from two experiments performed in triplicate.