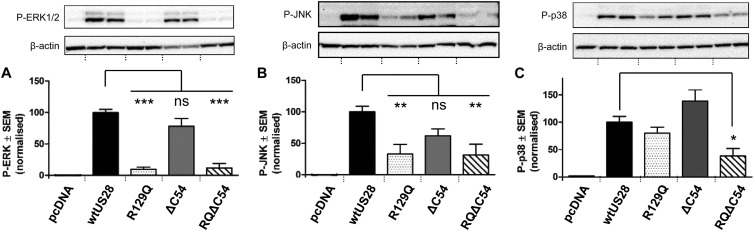
Fig 3.
HEK293 cells were transfected with 20 μg of the indicated HA-tagged US28 constructs or the pcDNA3 vector using calcium chloride precipitation. At 48 h after transfection, the cells were lysed on ice using radioimmunoprecipitation assay (RIPA) lysis buffer (Millipore). Samples were denatured at 92°C for 5 min and run on a 10% bis-Tris gel (Invitrogen) at 130 V for 1.5 h. The gel was blotted onto polyvinylidene difluoride (PVDF) membranes for 1.5 h and incubated with phosphospecific antibodies (Cell Signaling) against ERK (A) and JNK (B) simultaneously or p38 (C) overnight at 4°C. After incubation with horseradish peroxidase (HRP)-conjugated secondary antibodies, the membranes were developed using Immobilon Western chemiluminescent (Millipore) and quantified using AlphaView software; blots were stripped and reprobed for β-actin as a loading control. For each blot, background signal (pcDNA3) was subtracted and results were expressed relative to the mean signal for wt US28 (100%). Results are expressed as means ± standard errors of the means (SEM) from five independent experiments, each performed with duplicate samples. Representative blots are shown above the quantification graphs in each case. Asterisks denote significant differences between wt US28 and US28 mutants (analysis of variance [ANOVA] with Bonferroni posttest; *, P < 0.05; **, P < 0.01; ***, P < 0.001).