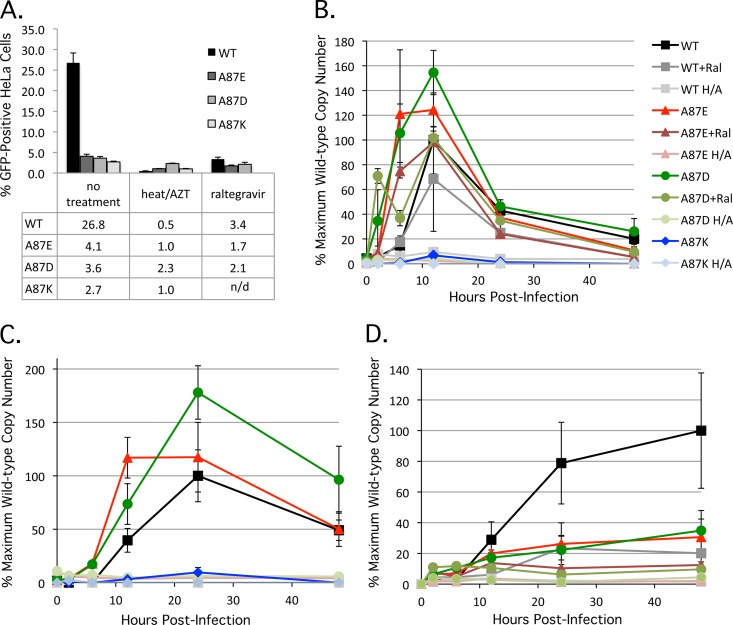
Fig 4.
Reverse transcription and integration of SIVmac239 variants. (A) Three independent infections of HeLa cells with wild-type and mutant SIVmac239 at a multiplicity of infection of approximately 0.5 were performed, and the percentage of infected, GFP-positive cells was assayed at 48 h postinfection, as described in the Fig. 2 legend. In some experiments, the viruses were incubated at 56°C for 1 h, and the cells were treated with 100 mM AZT (“heat/AZT”). In another set of experiments, 1 μM raltegravir was added to the infected cells. In the experiment shown, the value obtained for the heat-inactivated, AZT-treated A87D mutant is atypically high due to stochastic autofluorescence of the HeLa cells after treatment with 100 μM AZT. n/d, not determined. (B) To assess the production of late reverse transcripts, total DNA was extracted from infected HeLa cells at 2, 6, 12, 24, and 48 h postinfection. Late reverse transcript copy numbers were determined by quantitative real-time PCR (H/A, heat inactivation of virus plus AZT treatment of target cells, as described above; Ral, raltegravir treatment [1 μM] of target cells, as described above). The values shown represent the percentages of the maximum wild-type SIVmac239 value (7,245 copies/100 ng DNA at 12 h postinfection). (C) The production of 2-LTR circles was assessed as described in Materials and Methods. Heat inactivation of viruses plus AZT treatment of target cells was carried out as described above. The values shown represent the percentages of the maximum wild-type SIVmac239 value (552 copies/100 ng DNA at 24 h postinfection). (D) The production of integrated proviruses was assessed as described in Materials and Methods. Heat inactivation of viruses plus AZT treatment of target cells and raltegravir treatment of target cells were carried out as described above. The values shown represent the percentages of the maximum wild-type SIVmac239 value (4,725 copies/100 ng DNA at 48 h postinfection).