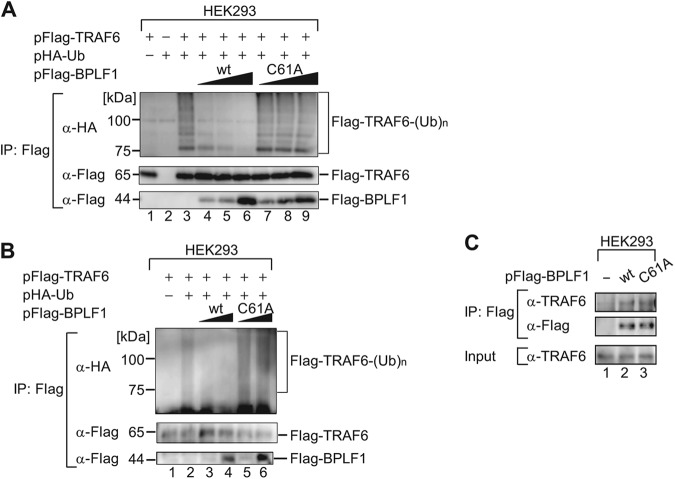
Fig 5.
BPLF1 interacts with and inhibits ubiquitination of TRAF6. (A) HEK293 cells cultured in 6-well plates were cotransfected with hemagglutinin (HA)-tagged Ub (2 μg/well) and TRAF6 (3 μg/well) expression plasmids and increasing quantities (0.1, 0.2, or 0.5 μg/well) of the designated BPLF1 expression plasmid. Cell lysates were prepared at 24 hpi and immunoprecipitated (IP) with anti-Flag antibodies, and ubiquitin conjugation of the TRAF6 protein was verified by immunoblotting with anti-HA antibodies. Production of exogenously expressed tagged proteins was verified with the indicated antibodies. The experiment shown is a representative of three independent experiments. (B) The conditions were basically the same as described for panel A except that cells were lysed with the denaturing lysis buffer containing 2% SDS followed by a 10-min incubation at 95°C. The amount of transfected BPLF1 expression plasmid was 0.1 or 0.5 μg/well. The experiment shown is a representative of three independent experiments. (C) HEK293 cells cultured in 6-well plates were transfected with an empty plasmid or designated BPLF1 (0.5 μg/well) expression plasmids. Cell lysates were prepared at 24 hpi and immunoprecipitated with anti-Flag antibodies, followed by immunoblot analysis with anti-TRAF6 antibodies. Production of exogenously expressed BPLF1 proteins was verified with anti-Flag antibody. The experiment shown is a representative of two independent experiments.