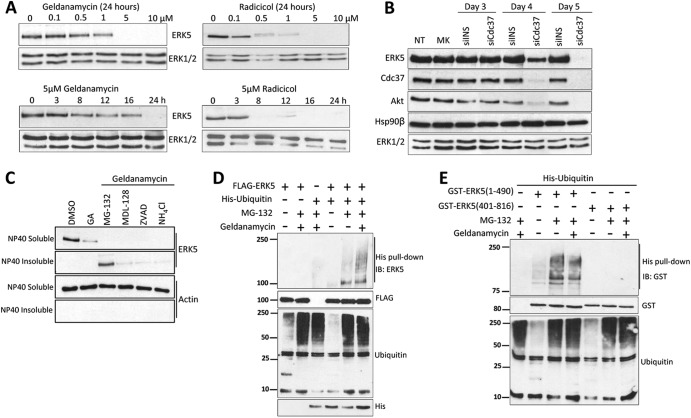
Fig 3.
Hsp90 inhibition or Cdc37 silencing induces loss of ERK5 protein by promoting its ubiquitylation and proteasome-mediated degradation. (A) Hsp90 inhibition induces loss of ERK5 expression. SH-SY5Y cells were treated with the indicated concentrations of geldanamycin or radicicol for 24 h (upper panels) or with a 5 μM concentration for the indicated times (lower panels). After the cells were subjected to lysis in buffer containing 1% SDS, protein levels were determined by immunoblotting. (B) Cdc37 silencing induces loss of ERK5 expression. HeLa cells transfected with 1 μg of Cdc37 siRNA or nonspecific siRNA (siINS) were lysed at the times indicated in buffer containing 1% SDS. Lysates were immunoblotted with the indicated antibodies. NT, no transfection; MK, mock transfection. (C) Treatment with the proteasome inhibitor and geldanamycin (GA) results in ERK5 accumulation in insoluble cellular fractions. SH-SY5Y cells were pretreated for 3 h with 20 μM proteasome inhibitor MG-132, 10 μM calpain inhibitor MDL-28170, 10 μM caspase inhibitor Z-VAD, or 20 mM ammonium chloride (inhibitor of lysosomal degradation), followed by 24 h of treatment with 5 μM geldanamycin. Cells were lysed in Nonidet P-40 buffer and centrifuged, and the NP-40 insoluble fraction (pellet) was solubilized in 1% SDS buffer. ERK5 and actin levels were analyzed by immunoblotting. (D and E) Treatment with geldanamycin induces ERK5 ubiquitylation. HEK293 cells overexpressing the indicated proteins were treated with MG-132 alone or with geldanamycin, and His-tagged ubiquitins were purified using Ni2+-NTA-agarose beads and immunoblotted (IB) for ERK5. ERK5 and total ubiquitin expression levels are shown in the middle and bottom panels, respectively. Levels of overexpressed free His-tagged ubiquitin are also shown in panel D. Similar results were obtained in three separate experiments.