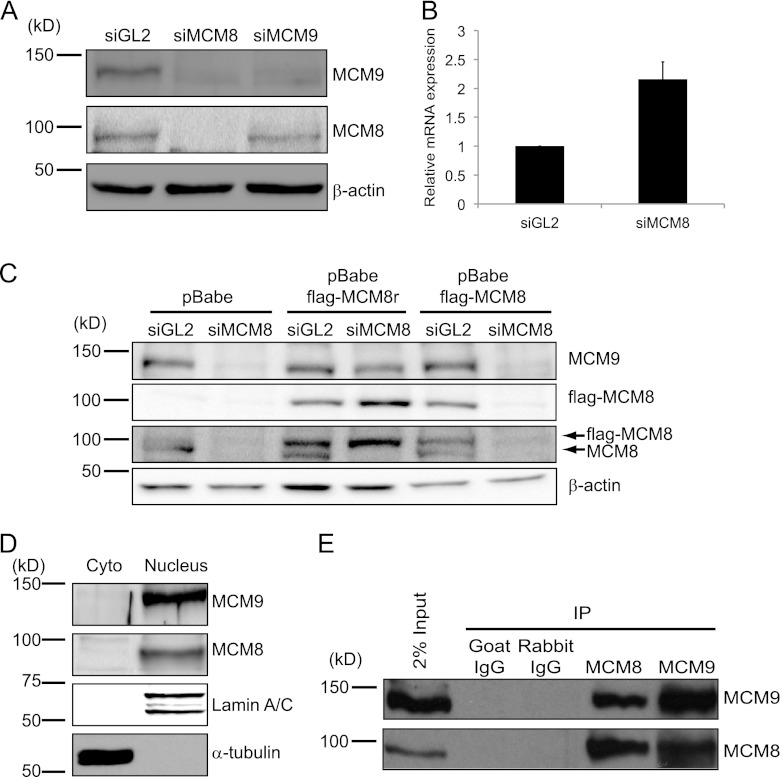
Fig 1.
MCM8 interacts with MCM9 and forms a stable nuclear complex. (A) Immunoblots of lysates of U2OS cells transfected with siRNA targeting the luciferase gene (siGL2, control) or the Mcm8 or Mcm9 genes (siMCM8 [siMCM8-ORF] and siMCM9, respectively). β-Actin protein serves as a loading control. (B) qRT-PCR of Mcm9 mRNA using total RNA from U2OS cells after siRNA transfection, normalized to GAPDH mRNA. Means + the standard deviations (SD) of triplicate measurements are shown. (C) Ectopic expression of siMCM8-resistant MCM8 protein (MCM8r) restores MCM9 levels in U2OS cells depleted of endogenous MCM8 protein. Wild-type and N-terminally Flag-tagged MCM8 (flag-MCM8) or siRNA resistant Flag-MCM8 protein (flag-MCM8r) were stably expressed in U2OS cells and transfected with siRNA targeting endogenous MCM8 or ectopic Flag-MCM8 but not ectopic MCM8r. Lysates were harvested 48 h after transfection and analyzed by SDS-PAGE and Western blotted with antibodies against the indicated proteins. The β-actin blot shows equal protein loading. (D) MCM8 and MCM9 are nuclear proteins. Immunoblots of cytoplasmic and nuclear proteins from 293T cells show that MCM8 and MCM9 are nuclear proteins. α-Tubulin and lamin A/C were used as controls of fractionation, respectively. Cyto, cytoplasmic proteins; Nucleus, nuclear proteins. (E) MCM8 associates with MCM9. Immunoprecipitates of endogenous MCM8 or MCM9 from 293T nuclear lysates coimmunoprecipitate MCM9 or MCM8, respectively.