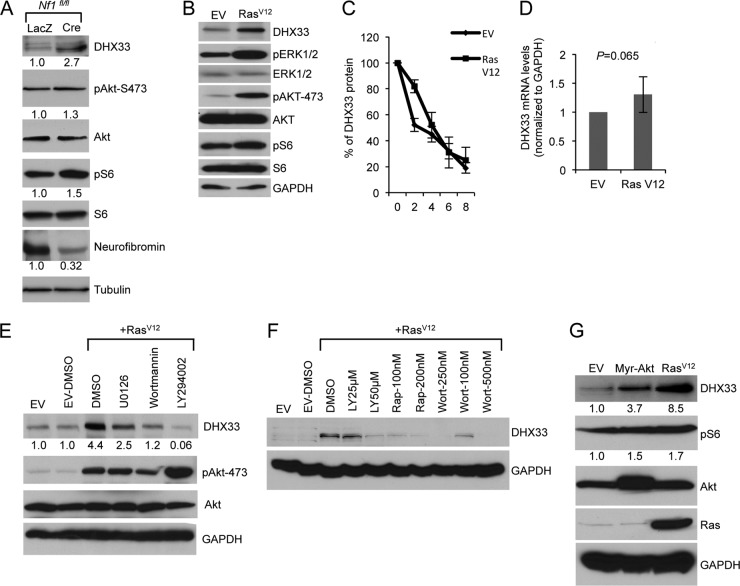
Fig 5.
Ras activity induces DHX33 protein expression. (A) Nf1fl/fl MEFs were infected with adenoviruses encoding either LacZ or Cre recombinase at a multiplicity of infection of 200. At 2 days postinfection, the cells were then serum starved for 72 h. Equal amount of cell lysates were subjected to Western blot analysis with the indicated antibodies. (B) Arf-null ear fibroblasts from 2-month-old mice were infected with retroviruses encoding either pBABE empty vector (EV) or pBABE-RasV12. At 3 days postinfection, infected cells were subjected to Western blot analysis with the indicated antibodies. (C) The above-mentioned cells were treated with cycloheximide at a concentration of 80 μg/ml for the indicated times. Protein extracts from the cells pelleted from the indicated time points was subjected to Western blot analysis. Signals of DHX33 protein was graphed after normalization to GAPDH control. Bars represent errors from two independent experiments. (D) Total RNA was isolated from the above-mentioned cells and changes of DHX33 mRNA levels were analyzed by qPCR with GAPDH as a control. P is derived from five separate experiments. (E) Arf-null ear fibroblasts were infected with empty vector or RasV12. At 3 days postinfection, the cells were treated with U0126 (20 μM), wortmannin (100 nM), or LY294002 (50 μM) for 24 h. Cell lysates were subjected to Western blot analysis with the indicated antibodies. (F) Arf-null cells infected with empty vector or RasV12 were treated with rapamycin, wortmannin, or LY294002 as indicated for 24 h. Cell lysates were prepared and analyzed for DHX33 protein levels with GAPDH as a loading control. (G) Arf-null MEFs were infected with retroviruses encoding myristoylated Akt (Myr-Akt), RasV12, or empty vector. Cell lysates were prepared at 4 days postinfection after puromycin selection and analyzed by Western blotting for DHX33, pAkt-473, Akt, and GAPDH protein levels. The fold change is indicated below identified blots.