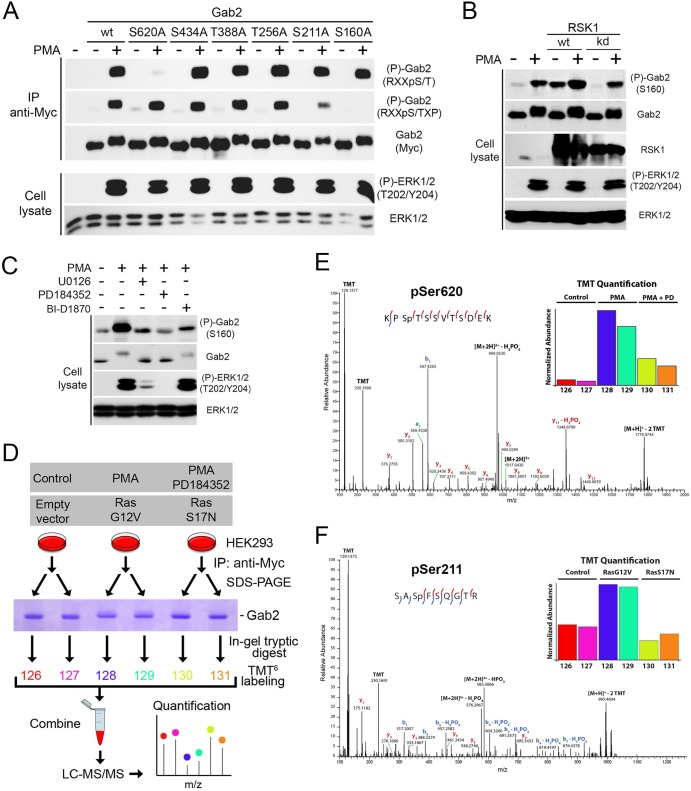
Fig 3.
Identification of Ser160, Ser211, and Ser620 as being regulated by Ras/MAPK signaling. (A) HEK293 cells were transfected with wt Gab2 or potential RSK phosphorylation site mutants (S160A, S211A, T256A, T388A, S434A, and S620A), serum starved overnight, and stimulated with PMA (100 ng/ml) for 30 min. Immunoprecipitated Myc-Gab2 was then assayed for phosphorylation with phosphomotif antibodies that recognize the RXXpS/T and RXXpS/TXP consensus motifs. (B) HEK293 cells were transfected with wt or a kinase-inactive form of RSK1, serum starved overnight, and stimulated with PMA (25 ng/ml) for 15 min. Endogenous Gab2 phosphorylation at Ser160 was determined with a phosphorylation site-specific antibody. (C) As for panel B, except that phosphorylation of endogenous Gab2 at Ser160 was monitored in HEK293 cells pretreated with UO126 (20 μM), PD184352 (10 μM), or BI-D1870 (10 μM) for 30 min prior to PMA stimulation. (D) Phosphorylation of murine Gab2 was confirmed via high-resolution MS/MS sequencing. Gab2 was immunoprecipitated in biological duplicate from cells following either mock treatment, treatment with PMA, or treatment with PMA and PD184352. In a separate experiment, cells were either transfected with an empty vector or different activated (G12V) and inactivated (S17N) alleles of Ras. After subsequent SDS-PAGE separation, bands corresponding to Gab2 were excised and proteins were digested in gel with trypsin followed by TMT labeling for relative quantitation. (E) The spectrum identifies a tryptic peptide bearing phosphorylation at Ser620, as well as TMT labels on both lysine side chains and the peptide N terminus; localization of the phosphorylation site is indicated by the presence of the y9 ion, which matches its theoretical mass to approximately 1 ppm. (Inset) Relative abundance of Ser620 across the six samples normalized with respect to overall Gab2 abundance. PD, PD184352. (F) The spectrum identifies a tryptic peptide bearing two TMT labels as well as phosphorylation of Ser211. Phosphorylation site localization is confirmed by the y5 and y6 ions and b1 and b2 ions, as well as the b3 and b4 ions with subsequent neutral loss of H3PO4. (Inset) Relative levels of Gab2 phosphorylation at Ser211, after normalization on the basis of overall Gab2 abundance. Red and blue lines within the peptide sequences indicate y and b ions, respectively.