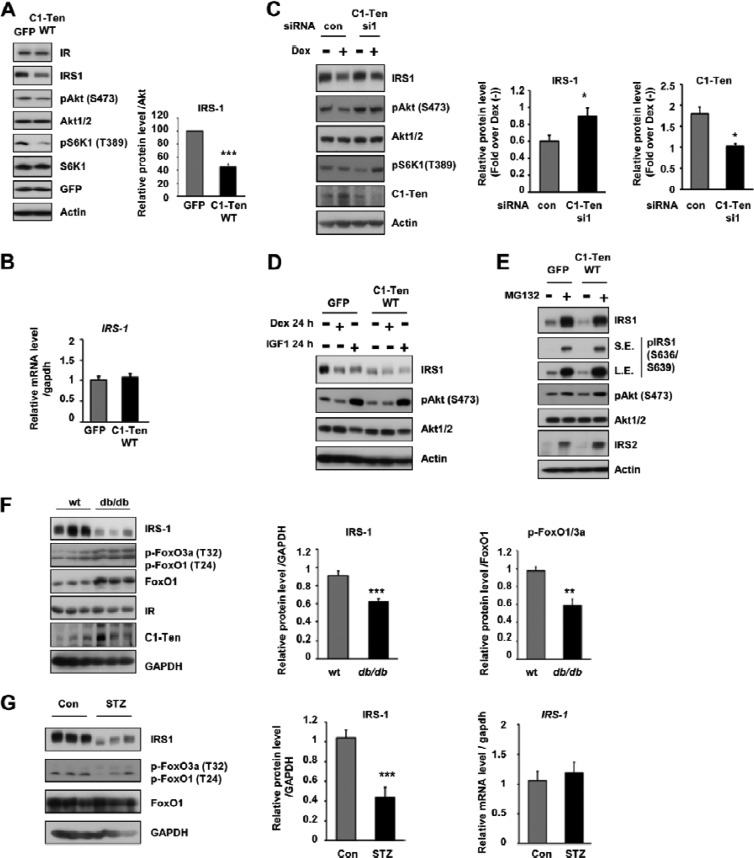
Fig 3.
C1-Ten-induced muscle atrophy via IRS-1 reduction. (A) Effect of C1-Ten on Akt/S6K1 signaling and IRS-1 levels. (Left) Myotubes at 8 days of differentiation were infected with Ad-GFP constructs for 48 h and subjected to immunoblotting; (right) levels of IRS-1 protein relative to those of Akt (n = 5). (B) The effect of C1-Ten on IRS-1 mRNA was determined by qRT-PCR (n = 3). (C) (Left) Effect of C1-Ten knockdown on glucocorticoid-induced IRS-1 reduction in myotubes. At 4 h posttransfection, myotubes were treated with 100 nM dexamethasone for 16 h. (Middle and right) protein levels presented as fold induction relative to dexamethasone-untreated [Dex (−)] sets (n = 5). (D) To determine the effect of dexamethasone or IGF-1 on IRS-1 and Akt, myotubes were infected with GFP- or C1-Ten-expressing virus vectors for 24 h and treated with 100 nM dexamethasone or 10 ng/ml IGF-1 for an additional 24 h. (E) The effects of MG132 on C1-Ten-induced IRS-1 reduction were determined by treating L6 myotubes with 20 μM MG132 for 2 h. (F) IRS-1 protein levels and FoxO activation in gastrocnemius muscles of db/db mice. IRS-1 and pFoxO1/3a protein levels were quantified relative to those of GAPDH (n = 6) and FoxO1 (n = 3), respectively. (G) Protein level (left and middle) and mRNA level (right) of IRS-1 in gastrocnemius muscles of streptozotocin (STZ)-treated rats. Streptozotocin (100 mg/kg of body weight) was dissolved in 0.1 M citrate buffer (pH 4.5) and injected into male rats (∼180 g) by the tail vein. Three days later, the rats were anesthetized and muscle tissue was harvested (n = 5). P values are compared to the control pair of fed rats. *, P < 0.05; **, P < 0.01; ***, P < 0.001.