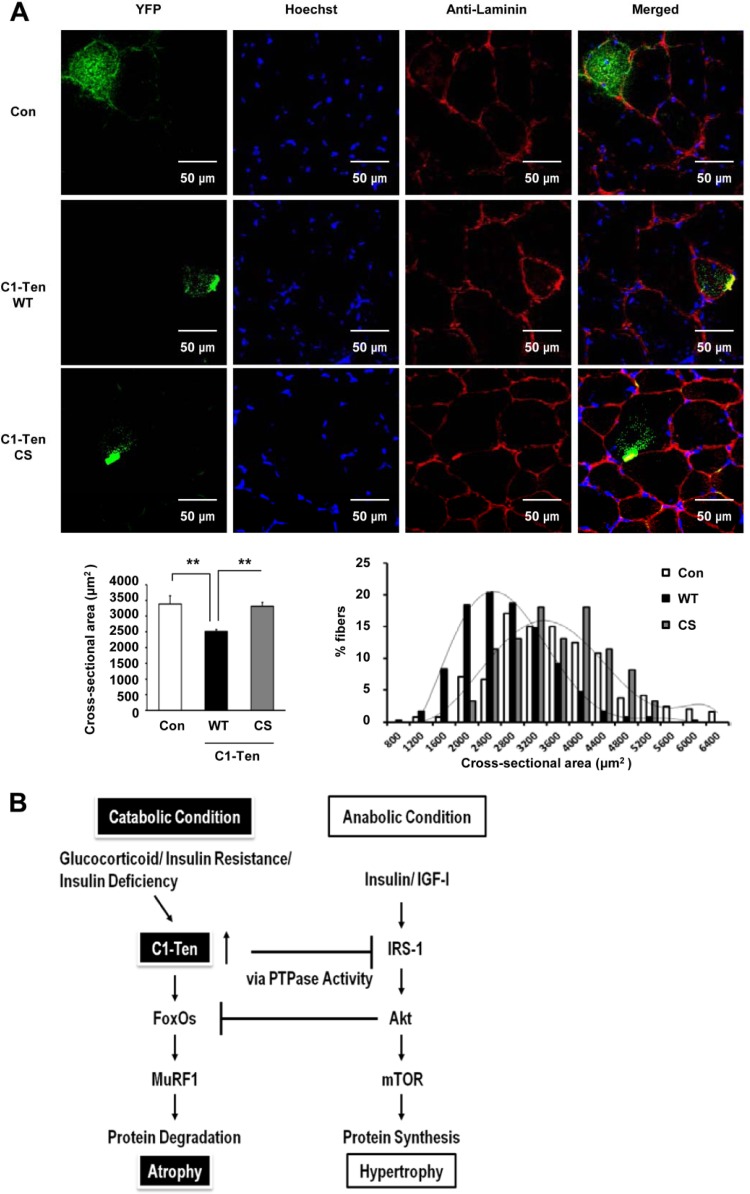
Fig 6.
C1-Ten PTPase-dependent regulation on adult muscle size. (A) Adult tibialis anterior muscles were transfected with YFP-, YFP–C1-Ten WT-, or YFP–C1-Ten CS mutant (C231S phosphatase-inactive mutant)-expressing vectors, and mice were killed after 12 days. Muscle cryosections (10 μm thick) were fixed with 4% paraformaldehyde at room temperature. After being rinsed with PBS, the cryosections were permeabilized with 0.2% Triton X-100 for 5 min and blocked with 3% bovine serum albumin. Cryosections were stained for Hoechst and laminin. Images were obtained with a confocal microscope (Olympus, Tokyo, Japan, or Leica sp5, Germany) and merged with the YFP signal. (Bottom left) Effect of C1-Ten expression on mean cross-sectional area (CSA). More than >200 fibers expressing each YFP construct were measured (n = 5). (Bottom right) Distribution of the CSA of fibers expressing YFP (control [Con]), YFP–C1-Ten WT (WT), or YFP–C1-Ten CS (CS). **, P < 0.01. (B) Schematic depicting skeletal muscle atrophy induced by C1-Ten under pathological conditions. The balance between anabolic and catabolic pathways is crucial for maintaining skeletal muscle mass. Anabolic pathways involving insulin and the IGF-1/IRS-1/Akt pathway not only promote protein synthesis (hypertrophy) but also suppress catabolic pathways, represented by FoxOs/MuRF1, leading to protein degradation (atrophy). Under pathological catabolic conditions, such as increased levels of glucocorticoids, insulin resistance, or insulin deficiency, C1-Ten is upregulated and induces IRS-1 degradation through its PTPase activity, subsequently downregulating the anabolic pathway and leading to atrophy.