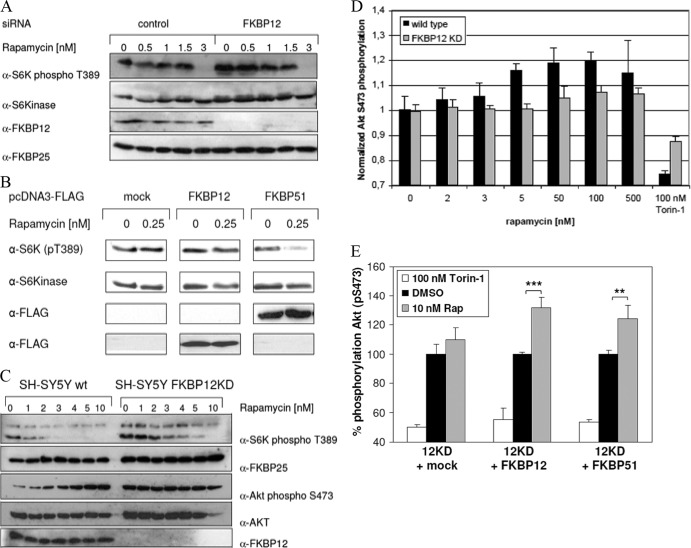
Fig 4.
Contributions of FKBPs to the cellular action of rapamycin. (A) Effect of rapamycin on S6K phosphorylation in FKBP12 knockdown HeLa cells. HeLa cells were transfected with siRNA for FKBP12 or nontargeting controls, starved, and stimulated with 10% FBS–100 nM insulin for 60 min in the presence of 0 to 3 nM rapamycin. Cells were lysed and analyzed by immunoblotting. FKBP25 probing served as an additional loading control. (B) Overexpression of FKBP12 or FKBP51 has different effects on S6 kinase phosphorylation. HeLa cells were transfected with an FKBP12 or FKBP51 overexpression vector, starved for 16 h, and stimulated with 10% FBS–100 nM insulin for 60 min in the absence or presence of 0.25 nM rapamycin. Thereafter, cells were lysed and subjected to SDS-PAGE and Western blotting. The membrane was probed with antibodies against the indicated proteins or antigens. Unrelated lanes between mock-transfected controls and FKBP12 and FKBP51 overexpression samples have been removed for clarity. (C) In FKBP12 knockdown cells, rapamycin-induced Akt hyperphosphorylation is blunted. Wild-type (wt) and FKBP12 knockdown neuroblastoma cells were starved for 24 h and stimulated with 10% FCS–100 nM insulin for 60 min in the presence of 0 to 10 nM rapamycin. Cells were lysed and subjected to SDS-PAGE and immunoblotting. S6 kinase and Akt phosphorylation was assessed with the appropriate phospho-specific antibodies. (D) FKBP12 is necessary for rapamycin-induced Akt hyperphosphorylation. Wild-type and FKBP12 knockdown SH-SY5Y neuroblastoma cells were starved for 24 h and stimulated with 10% FCS–100 nM insulin for 60 min in the presence of 0 to 500 nM rapamycin or 100 nM torin-1. Cells were lysed, and Akt S473 phosphorylation was assessed with a phospho-antibody-based FRET assay. Mean values of two independent duplicates are shown. Values were normalized to 1 for 0 nM rapamycin for each cell type. (E) FKBP51 can replace FKBP12 in enabling rapamycin (Rap)-induced Akt hyperphosphorylation. FKBP12 knockdown SH-SY5Y cells were transfected with FKBP12, FKBP51, or a control plasmid and treated 24 h later with 10 nM rapamycin or 100 nM torin-1 for 60 min. Cellular Akt S473 phosphorylation was determined with a homogeneous time-resolved FRET assay. Mean values of three independent data points are shown. Results were normalized to those of dimethyl sulfoxide (DMSO)-treated controls (100%) for each transfection condition. ***, P < 0.001; **, P < 0.01.