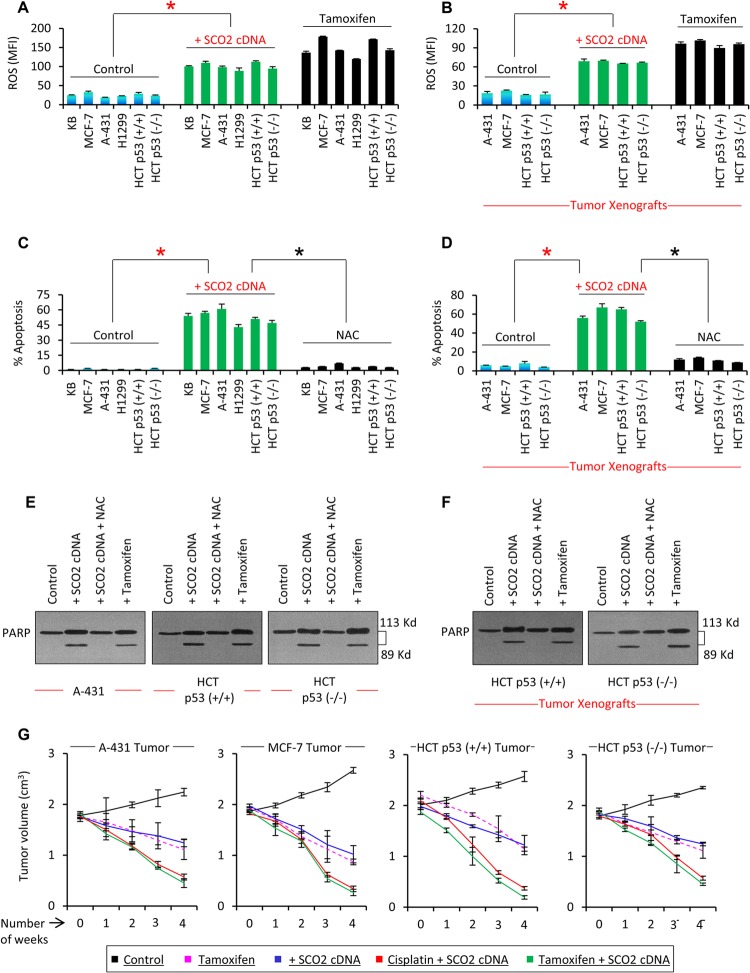
Fig 2.
SCO2 induces p53 downstream apoptosis by generating ROS. (A) The effect of SCO2 on cellular ROS was observed in p53 wild-type cells (KB, MCF-7, and HCT p53+/+), p53 mutant cells (A-431), and p53 null cells (H1299). SCO2-overexpressing cells (bars 7 to 12) showed a >5-fold increase in the ROS content in comparison with control cells (bars 1 to 6). Tamoxifen treatment served as a positive control (an asterisk represents a significant difference between control and SCO2-transfected groups [P < 0.029]; n = 7; error bars represent standard deviations determined by analysis of variance). MFI, mean fluorescence intensity. (B) The effect of SCO2 on cellular ROS was also analyzed in tumor xenografts. p53 mutant (A-431), p53 null (HCT p53−/−), and p53 wild-type (MCF-7 and HCT p53+/+) tumor xenografts were ectopically expressed with SCO2 cDNA, which resulted in a 4-fold increase in ROS content (bars 5 to 8). Tamoxifen treatment served as a positive control (∗, P < 0.045; n = 7; error bars represent standard deviations determined by analysis of variance). (C) The effect of SCO2 overexpression on cellular apoptosis was observed in p53 wild-type cells (KB, MCF-7, and HCT p53+/+), p53 mutant cells (A-431), and p53 null cells (H1299) by annexin V staining and flow cytometry. A significant increase in the apoptotic fraction of SCO2-overexpressing cells was seen irrespective of the p53 gene status (bars 7 to 12). ROS quenching via NAC abolished SCO2-mediated apoptosis (bars 13 to 18). Untreated cells served as negative controls, while tamoxifen served as a positive control (an asterisk represents a significant difference between control and SCO2-treated cells and between SCO2-treated and ROS-quenched cells [P < 0.021 and P < 0.025]; n = 7; error bars represent standard deviations determined by analysis of variance). (D) The role of SCO2 in inducing apoptosis was observed in p53 mutant (A-431), p53 null (HCT p53−/−), and p53 wild-type (MCF-7 and HCT p53+/+) tumor xenografts by using a FLIVO in vivo apoptosis kit (23). A significant increase in the apoptotic fraction of SCO2-transfected xenografts was seen in comparison with control tumors (bars 5 to 8), and ROS quenching via NAC suppressed SCO2-induced apoptosis (∗, P < 0.034 and P < 0.039; n = 7; error bars represent standard deviations determined by analysis of variance). (E) The effect of SCO2 overexpression on cleavage of PARP was observed in vitro for A-431, HCT p53+/+, and HCT p53−/− cancer cells. SCO2 cDNA was expressed ectopically in cancer cells in the presence and absence of NAC. Results show that ectopic expression of SCO2 resulted in cleavage of PARP in all the cancer cell lines (lane 2). Quenching of ROS via NAC abolished PARP cleavage (lane 3). Tamoxifen treatment served as a positive control and cleaved PARP (n = 5). (F) The effect of exogenous addition of SCO2 on PARP cleavage in p53+/+ and HCT p53−/− tumor xenografts was observed in the presence and absence of NAC. Ectopic expression of SCO2 resulted in PARP cleavage (lane 2). NAC abolished PARP cleavage (lane 3). Untreated cells and tamoxifen-treated cells served as controls (n = 4). (G) The potential of SCO2 to induce tumor regression was observed in A-431, MCF-7, HCT p53+/+, and HCT p53−/− tumor xenografts. Results show that exogenous addition of SCO2 cDNA led to a significant reduction in the size of tumor xenografts in 4 weeks. Combinatorial therapy of SCO2 and cisplatin and of SCO2 and tamoxifen caused a >85% regression of the tumor in 4 weeks. Untreated tumors served as controls; tamoxifen treatment served as a positive control (n = 10; error bars represent standard deviations determined by analysis of variance).