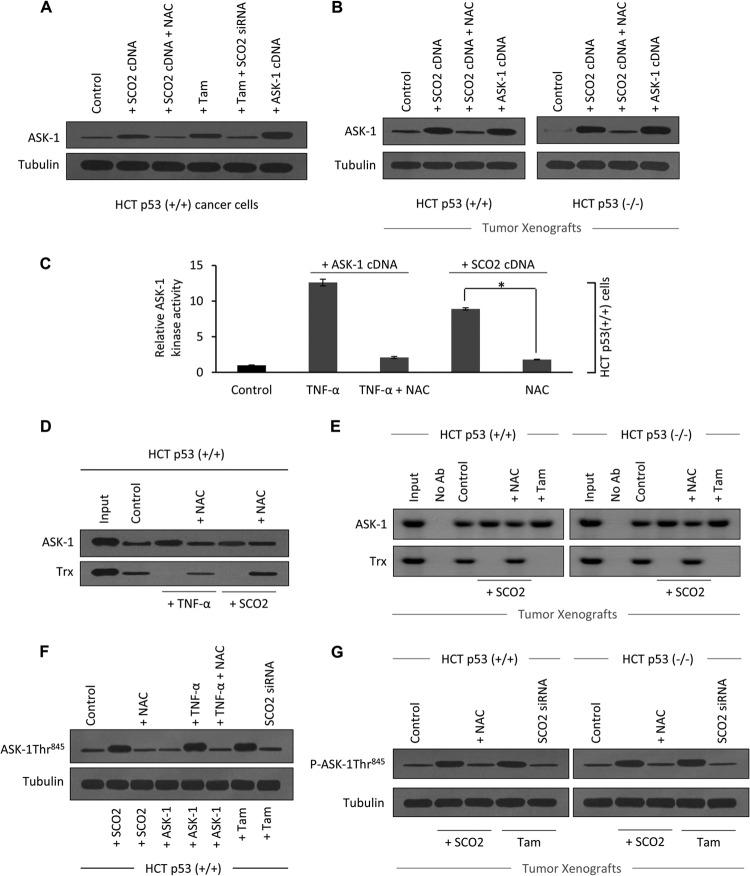
Fig 5.
SCO2 induces dissociation of the ASK-1–Trx complex. (A) The role of SCO2 and SCO2-induced ROS in the regulation of expression of ASK-1 protein in HCT (p53+/+) cells was studied. Untreated cells were used as controls (lane 1). HCT cells ectopically expressed with SCO2 cDNA (1 μg) resulted in a significant upregulation of the ASK-1 protein (lane 2). ROS quenching by NAC treatment resulted in a significant decrease in the ASK-1 protein level (lane 3). Tamoxifen was used as a positive control (lane 4). Silencing of the SCO2 gene by SCO2 siRNA reversed the tamoxifen-induced increase in the ASK-1 protein expression level (lane 5). ASK-1 cDNA transfection was used as a positive control (lane 6) (n = 5). (B) The role of SCO2 and SCO2-induced ROS in expression of ASK-1 protein was observed by using HCT p53+/+ and HCT p53−/− tumor xenografts. Western blot analysis showed increased ASK-1 protein expression levels in SCO2-overexpressed tumors (lane 2). ROS quenching via NAC reversed SCO2-induced ASK-1 upregulation (lane 3). Transfected ASK-1 cDNA in tissue was used as a positive control (lane 4) (n = 4). (C) The role of SCO2 in the regulation of ASK-1 kinase activity was studied in HCT p53+/+ cells, using MBP as a substrate. TNF-α, which is a known activator of ASK-1 kinase activity, was used as a positive control. The level of ASK-1 kinase activity was very low in the untreated cells (bar 1), and treatment with TNF-α significantly increased the ASK-1 kinase activity (bar 2). ROS quenching through NAC reversed the TNF-α-induced increase in the ASK-1 kinase activity (bar 3). The exogenous addition of SCO2 cDNA (1 μg) in HCT cells resulted in a significant increase in ASK-1 kinase activity (bar 4), which was reversed upon ROS quenching (bar 5) (n = 5; an asterisk represents a significant difference between bars 4 and 5 [P < 0.029]; error bars represent standard deviations determined by analysis of variance). (D) The role of SCO2 in inducing the dissociation of the ASK-1–Trx complex was studied by using coimmunoprecipitation in HCT p53+/+ cells. ASK-1 protein was pulled down by using anti-ASK-1 Ab, and Western blots were developed by using anti-ASK-1 and anti-Trx Abs. In HCT p53+/+ cells, ASK-1 bound to its inhibitor Trx (lane 2). TNF-α treatment broke the interaction between ASK-1 and Trx (lane 3), and ROS quenching restored their interaction (lane 4). Exogenous addition of SCO2 cDNA (1 μg) resulted in dissociation of ASK-1 from the Trx protein (lane 5), and ROS quenching restored their interaction (lane 6) (n = 4). (E) Exogenous addition of SCO2 results in dissociation of the ASK-1–Trx complex in HCT p53+/+ and HCT p53−/− tumor xenografts. Exogenous addition of SCO2 cDNA resulted in ASK-1–Trx protein dissociation (lane 4). ROS quenching by NAC reversed the dissociation (lane 5). Input, IPP with no Ab. Untreated cells were used as controls (n = 4). (F) The role of SCO2 in inducing the phosphorylation of ASK-1 at the Thr845 residue was studied by using Western blotting of HCT p53+/+ cells. Control HCT cells showed minimal Thr845 phosphorylation (lane 1). Exogenous addition of SCO2 cDNA resulted in a significant increase in ASK-1 Thr845 residue phosphorylation (lane 2), and ROS quenching abolished this phosphorylation (lane 3). TNF-α also induced phosphorylation, which was reversed upon ROS quenching (lanes 5 and 6). Tamoxifen induced phosphorylation (lane 7), and SCO2 siRNA abolished it (lane 8) (n = 5). (G) Similarly, SCO2 was observed to induce the phosphorylation of ASK-1 at the Thr845 residue in HCT p53+/+ and HCT p53−/− tumor xenografts (lane 2), and ROS quenching reversed it (lane 3). Tamoxifen treatment was used as a positive control (n = 4).