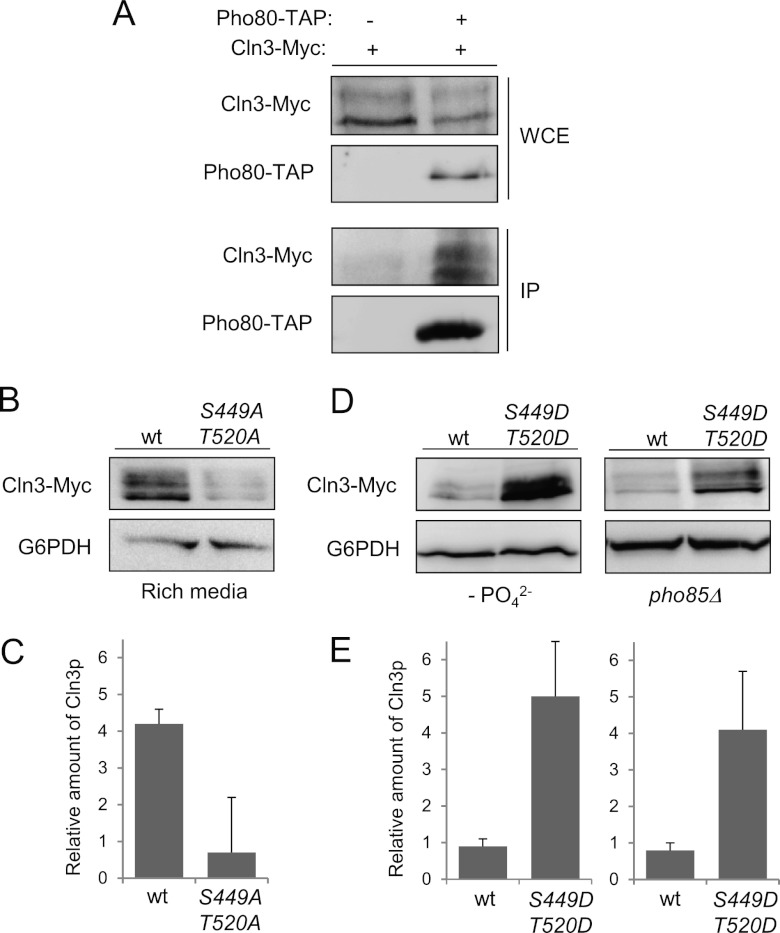
Fig 7.
In vivo phosphorylation of S449 and T520 is essential to maintain Cln3 levels. (A) Pho80 and Cln3 interact in vivo. Yeast extracts (WCE [whole-cell extracts]) containing untagged Pho80 or Pho80-TAP were pulled down with IgG-Sepharose. Tagged Cln3-Myc (from its chromosomal locus) was detected using specific antibodies (IP [immunoprecipitate]). (B) Alanine replacement of S449 and T520 destabilizes Cln3. Wild-type cells were transformed with a centromeric plasmid bearing a Cln3-Myc or a 2-Ala (S449A T520A) mutant version and grown for 4 h in rich medium. The amount of Cln3 was determined by immunoblotting. (C) Quantification of experiment whose results are shown in panel B. Data ± standard deviations of three independent experiments are shown. (D) Aspartic acid replacement of S449 and T520 stabilizes Cln3. Wild-type (in phosphate-deficient medium) or pho85Δ (in phosphate-rich medium) cells bearing a plasmid with different versions of CLN3 were grown exponentially for 4 h. Cln3 levels were analyzed by immunoblotting. To simplify the nomenclature, S449D means a double substitution of Asp into the Ser 449 and the previous residue to mimic the double-negative charge that represents the phosphate group (see Materials and Methods), and the same is true for T520D. (E) Quantification of experiments whose results are shown in panel D. Data ± standard deviations of three independent experiments are shown.