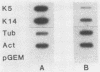
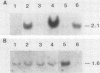
Abstract
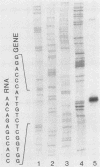
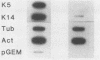
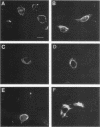
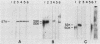
The mitotically active basal layers of most stratified squamous epithelia express 10 to 30% of their total protein as keratin. The two keratins specifically expressed in these cells are the type II keratin K5 (58 kilodaltons) and its corresponding partner, type I keratin K14 (50 kilodaltons), both of which are essential for the formation of 8-nm filaments. Dissecting the molecular mechanisms underlying the coordinate regulation of the two keratins is an important first step in understanding epidermal differentiation and in designing promoters that will enable delivery and expression of foreign gene products in stratified squamous epithelia, e.g., skin. Previously, we reported the sequence of the gene encoding human K14 (D. Marchuk, S. McCrohon, and E. Fuchs, Cell 39:491-498, 1984; Marchuk et al., Proc. Natl. Acad. Sci. USA 82:1609-1613, 1985). We have now isolated and characterized the gene encoding human K5. The sequence of the coding portion of this gene matched perfectly with that of a partial K5 cDNA sequence obtained from a cultured human epidermal library (R. Lersch and E. Fuchs, Mol. Cell. Biol. 8:486-493, 1988), and gene transfection studies indicated that the gene is functional. Nuclear runoff experiments demonstrated that the K5 and K14 genes were both transcribed at dramatically higher levels in cultured human epidermal cells than in fibroblasts, indicating that at least part of the regulation of the expression of this keratin pair is at the transcriptional level. When the K5 gene was transfected transiently into NIH 3T3 fibroblasts, foreign expression of the gene caused the appearance of endogenous mouse K14 and the subsequent formation of a keratin filament array in the cells. In this case, transcriptional changes did not appear to be involved in the regulation, suggesting that there may be multiple control mechanisms underlying the pairwise expression of keratins.
Full text
PDF

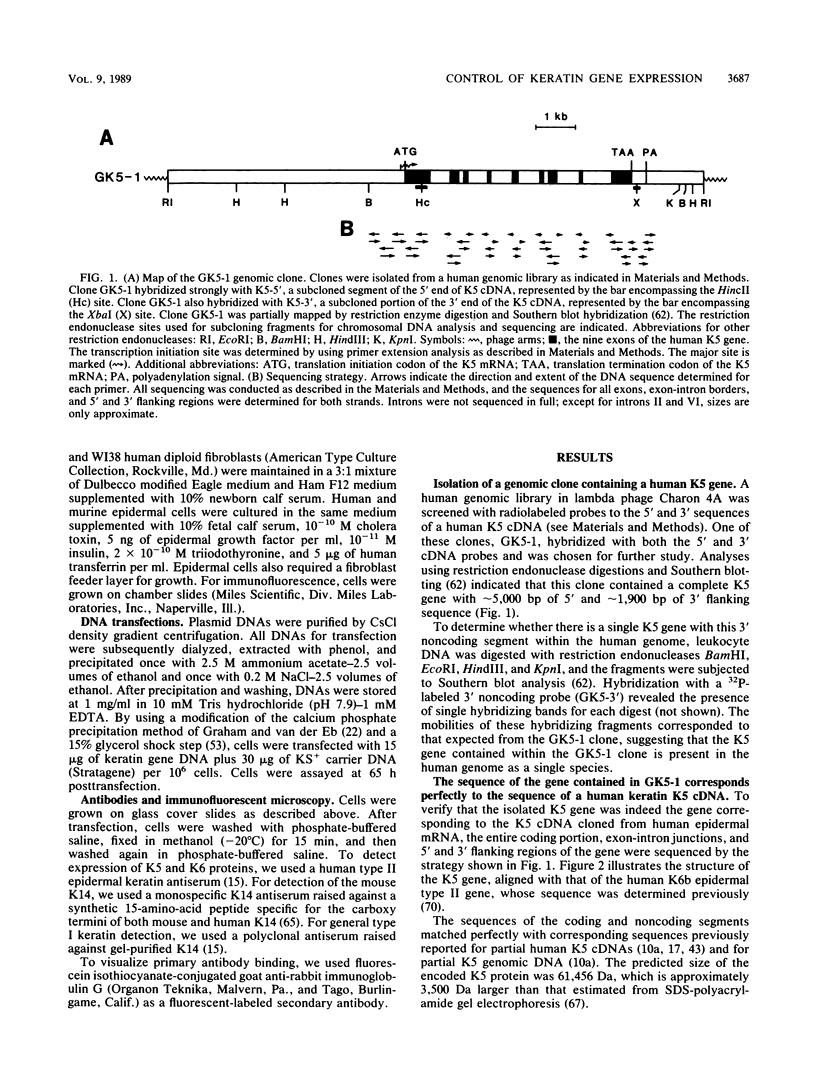
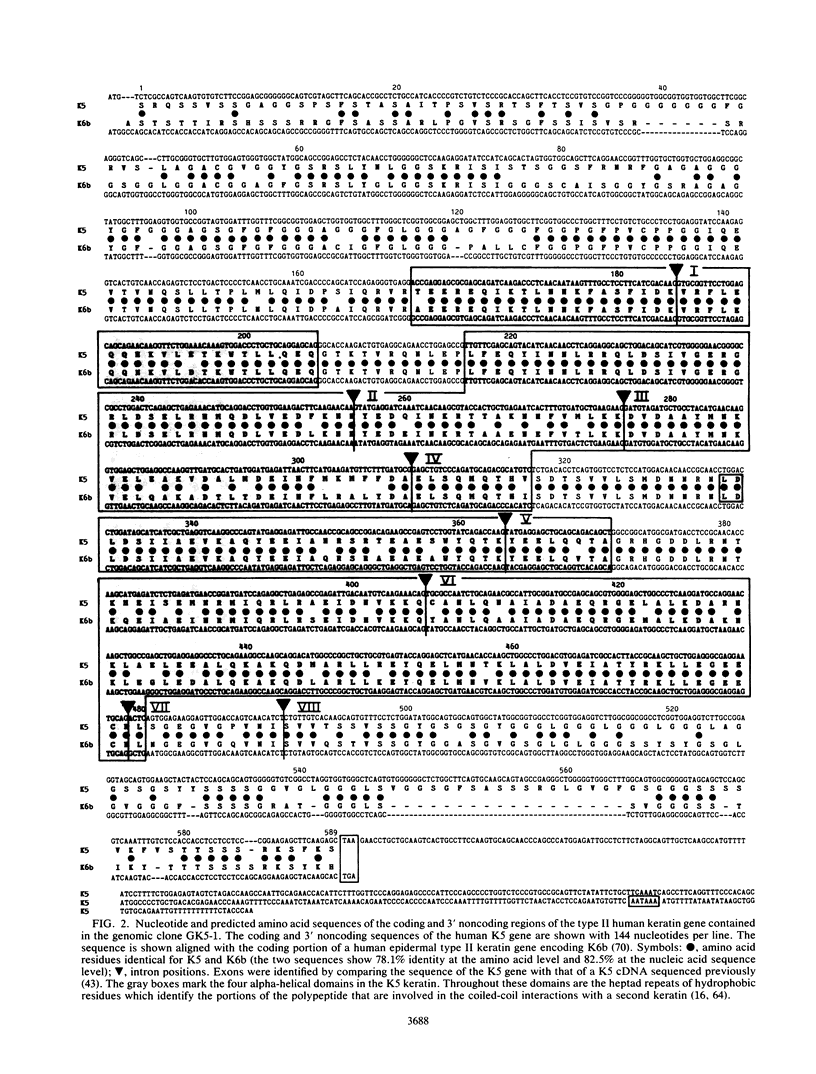




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bader B. L., Magin T. M., Hatzfeld M., Franke W. W. Amino acid sequence and gene organization of cytokeratin no. 19, an exceptional tail-less intermediate filament protein. EMBO J. 1986 Aug;5(8):1865–1875. doi: 10.1002/j.1460-2075.1986.tb04438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Blessing M., Zentgraf H., Jorcano J. L. Differentially expressed bovine cytokeratin genes. Analysis of gene linkage and evolutionary conservation of 5'-upstream sequences. EMBO J. 1987 Mar;6(3):567–575. doi: 10.1002/j.1460-2075.1987.tb04792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of beta-turns. Biophys J. 1979 Jun;26(3):367–383. doi: 10.1016/S0006-3495(79)85259-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of the secondary structure of proteins from their amino acid sequence. Adv Enzymol Relat Areas Mol Biol. 1978;47:45–148. doi: 10.1002/9780470122921.ch2. [DOI] [PubMed] [Google Scholar]
- Cowan N. J., Dobner P. R., Fuchs E. V., Cleveland D. W. Expression of human alpha-tubulin genes: interspecies conservation of 3' untranslated regions. Mol Cell Biol. 1983 Oct;3(10):1738–1745. doi: 10.1128/mcb.3.10.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale B. A., Holbrook K. A., Kimball J. R., Hoff M., Sun T. T. Expression of epidermal keratins and filaggrin during human fetal skin development. J Cell Biol. 1985 Oct;101(4):1257–1269. doi: 10.1083/jcb.101.4.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darmon M. Co-expression of specific acid and basic cytokeratins in teratocarcinoma-derived fibroblasts treated with 5-azacytidine. Dev Biol. 1985 Jul;110(1):47–52. doi: 10.1016/0012-1606(85)90062-4. [DOI] [PubMed] [Google Scholar]
- Eckert R. L., Rorke E. A. The sequence of the human epidermal 58-kD (#5) type II keratin reveals an absence of 5' upstream sequence conservation between coexpressed epidermal keratins. DNA. 1988 Jun;7(5):337–345. doi: 10.1089/dna.1.1988.7.337. [DOI] [PubMed] [Google Scholar]
- Eichner R., Bonitz P., Sun T. T. Classification of epidermal keratins according to their immunoreactivity, isoelectric point, and mode of expression. J Cell Biol. 1984 Apr;98(4):1388–1396. doi: 10.1083/jcb.98.4.1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichner R., Sun T. T., Aebi U. The role of keratin subfamilies and keratin pairs in the formation of human epidermal intermediate filaments. J Cell Biol. 1986 May;102(5):1767–1777. doi: 10.1083/jcb.102.5.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs E. V., Coppock S. M., Green H., Cleveland D. W. Two distinct classes of keratin genes and their evolutionary significance. Cell. 1981 Nov;27(1 Pt 2):75–84. doi: 10.1016/0092-8674(81)90362-7. [DOI] [PubMed] [Google Scholar]
- Fuchs E., Green H. Changes in keratin gene expression during terminal differentiation of the keratinocyte. Cell. 1980 Apr;19(4):1033–1042. doi: 10.1016/0092-8674(80)90094-x. [DOI] [PubMed] [Google Scholar]
- Fuchs E., Marchuk D. Type I and type II keratins have evolved from lower eukaryotes to form the epidermal intermediate filaments in mammalian skin. Proc Natl Acad Sci U S A. 1983 Oct;80(19):5857–5861. doi: 10.1073/pnas.80.19.5857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs E., Marchuk D. Type I and type II keratins have evolved from lower eukaryotes to form the epidermal intermediate filaments in mammalian skin. Proc Natl Acad Sci U S A. 1983 Oct;80(19):5857–5861. doi: 10.1073/pnas.80.19.5857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs E., Tyner A. L., Giudice G. J., Marchuk D., RayChaudhury A., Rosenberg M. The human keratin genes and their differential expression. Curr Top Dev Biol. 1987;22:5–34. doi: 10.1016/s0070-2153(08)60097-6. [DOI] [PubMed] [Google Scholar]
- Galup C., Darmon M. Y. Isolation and characterization of a cDNA clone coding for human epidermal keratin K5. Sequence of the carboxyterminal half of this keratin. J Invest Dermatol. 1988 Jul;91(1):39–42. doi: 10.1111/1523-1747.ep12463286. [DOI] [PubMed] [Google Scholar]
- Geisler N., Kaufmann E., Weber K. Antiparallel orientation of the two double-stranded coiled-coils in the tetrameric protofilament unit of intermediate filaments. J Mol Biol. 1985 Mar 5;182(1):173–177. doi: 10.1016/0022-2836(85)90035-x. [DOI] [PubMed] [Google Scholar]
- Giudice G. J., Fuchs E. The transfection of epidermal keratin genes into fibroblasts and simple epithelial cells: evidence for inducing a type I keratin by a type II gene. Cell. 1987 Feb 13;48(3):453–463. doi: 10.1016/0092-8674(87)90196-6. [DOI] [PubMed] [Google Scholar]
- Glass C., Fuchs E. Isolation, sequence, and differential expression of a human K7 gene in simple epithelial cells. J Cell Biol. 1988 Oct;107(4):1337–1350. doi: 10.1083/jcb.107.4.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass C., Kim K. H., Fuchs E. Sequence and expression of a human type II mesothelial keratin. J Cell Biol. 1985 Dec;101(6):2366–2373. doi: 10.1083/jcb.101.6.2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Greenberg M. E., Ziff E. B. Stimulation of 3T3 cells induces transcription of the c-fos proto-oncogene. Nature. 1984 Oct 4;311(5985):433–438. doi: 10.1038/311433a0. [DOI] [PubMed] [Google Scholar]
- Groudine M., Peretz M., Weintraub H. Transcriptional regulation of hemoglobin switching in chicken embryos. Mol Cell Biol. 1981 Mar;1(3):281–288. doi: 10.1128/mcb.1.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanukoglu I., Fuchs E. The cDNA sequence of a Type II cytoskeletal keratin reveals constant and variable structural domains among keratins. Cell. 1983 Jul;33(3):915–924. doi: 10.1016/0092-8674(83)90034-x. [DOI] [PubMed] [Google Scholar]
- Hanukoglu I., Fuchs E. The cDNA sequence of a human epidermal keratin: divergence of sequence but conservation of structure among intermediate filament proteins. Cell. 1982 Nov;31(1):243–252. doi: 10.1016/0092-8674(82)90424-x. [DOI] [PubMed] [Google Scholar]
- Hanukoglu I., Tanese N., Fuchs E. Complementary DNA sequence of a human cytoplasmic actin. Interspecies divergence of 3' non-coding regions. J Mol Biol. 1983 Feb 5;163(4):673–678. doi: 10.1016/0022-2836(83)90117-1. [DOI] [PubMed] [Google Scholar]
- Hatzfeld M., Franke W. W. Pair formation and promiscuity of cytokeratins: formation in vitro of heterotypic complexes and intermediate-sized filaments by homologous and heterologous recombinations of purified polypeptides. J Cell Biol. 1985 Nov;101(5 Pt 1):1826–1841. doi: 10.1083/jcb.101.5.1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson L. D., Idler W. W., Zhou X. M., Roop D. R., Steinert P. M. Structure of a gene for the human epidermal 67-kDa keratin. Proc Natl Acad Sci U S A. 1985 Apr;82(7):1896–1900. doi: 10.1073/pnas.82.7.1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas E., Sargent T. D., Dawid I. B. Epidermal keratin gene expressed in embryos of Xenopus laevis. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5413–5417. doi: 10.1073/pnas.82.16.5413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones N. C., Rigby P. W., Ziff E. B. Trans-acting protein factors and the regulation of eukaryotic transcription: lessons from studies on DNA tumor viruses. Genes Dev. 1988 Mar;2(3):267–281. doi: 10.1101/gad.2.3.267. [DOI] [PubMed] [Google Scholar]
- Karlsson S., Nienhuis A. W. Developmental regulation of human globin genes. Annu Rev Biochem. 1985;54:1071–1108. doi: 10.1146/annurev.bi.54.070185.005231. [DOI] [PubMed] [Google Scholar]
- Kim K. H., Rheinwald J. G., Fuchs E. V. Tissue specificity of epithelial keratins: differential expression of mRNAs from two multigene families. Mol Cell Biol. 1983 Apr;3(4):495–502. doi: 10.1128/mcb.3.4.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp B., Rentrop M., Schweizer J., Winter H. Three cDNA sequences of mouse type I keratins. Cellular localization of the mRNAs in normal and hyperproliferative tissues. J Biol Chem. 1987 Jan 15;262(2):938–945. [PubMed] [Google Scholar]
- Kopan R., Fuchs E. A new look into an old problem: keratins as tools to investigate determination, morphogenesis, and differentiation in skin. Genes Dev. 1989 Jan;3(1):1–15. doi: 10.1101/gad.3.1.1. [DOI] [PubMed] [Google Scholar]
- Kopan R., Traska G., Fuchs E. Retinoids as important regulators of terminal differentiation: examining keratin expression in individual epidermal cells at various stages of keratinization. J Cell Biol. 1987 Jul;105(1):427–440. doi: 10.1083/jcb.105.1.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg T. M., Schafer M. P., Cheng C. K., Filpula D., Flaherty P., Steinert P. M., Roop D. R. Organization of a type I keratin gene. Evidence for evolution of intermediate filaments from a common ancestral gene. J Biol Chem. 1985 May 25;260(10):5867–5870. [PubMed] [Google Scholar]
- Kulesh D. A., Ceceña G., Darmon Y. M., Vasseur M., Oshima R. G. Posttranslational regulation of keratins: degradation of mouse and human keratins 18 and 8. Mol Cell Biol. 1989 Apr;9(4):1553–1565. doi: 10.1128/mcb.9.4.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulesh D. A., Oshima R. G. Cloning of the human keratin 18 gene and its expression in nonepithelial mouse cells. Mol Cell Biol. 1988 Apr;8(4):1540–1550. doi: 10.1128/mcb.8.4.1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land H., Chen A. C., Morgenstern J. P., Parada L. F., Weinberg R. A. Behavior of myc and ras oncogenes in transformation of rat embryo fibroblasts. Mol Cell Biol. 1986 Jun;6(6):1917–1925. doi: 10.1128/mcb.6.6.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee L. D., Baden H. P. Organisation of the polypeptide chains in mammalian keratin. Nature. 1976 Nov 25;264(5584):377–379. doi: 10.1038/264377a0. [DOI] [PubMed] [Google Scholar]
- Lee W., Haslinger A., Karin M., Tjian R. Activation of transcription by two factors that bind promoter and enhancer sequences of the human metallothionein gene and SV40. Nature. 1987 Jan 22;325(6102):368–372. doi: 10.1038/325368a0. [DOI] [PubMed] [Google Scholar]
- Lersch R., Fuchs E. Sequence and expression of a type II keratin, K5, in human epidermal cells. Mol Cell Biol. 1988 Jan;8(1):486–493. doi: 10.1128/mcb.8.1.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magin T. M., Jorcano J. L., Franke W. W. Translational products of mRNAs coding for non-epidermal cytokeratins. EMBO J. 1983;2(8):1387–1392. doi: 10.1002/j.1460-2075.1983.tb01596.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansbridge J. N., Knapp A. M. Changes in keratinocyte maturation during wound healing. J Invest Dermatol. 1987 Sep;89(3):253–263. doi: 10.1111/1523-1747.ep12471216. [DOI] [PubMed] [Google Scholar]
- Marchuk D., McCrohon S., Fuchs E. Complete sequence of a gene encoding a human type I keratin: sequences homologous to enhancer elements in the regulatory region of the gene. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1609–1613. doi: 10.1073/pnas.82.6.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchuk D., McCrohon S., Fuchs E. Remarkable conservation of structure among intermediate filament genes. Cell. 1984 Dec;39(3 Pt 2):491–498. doi: 10.1016/0092-8674(84)90456-2. [DOI] [PubMed] [Google Scholar]
- McKnight S. L., Kingsbury R. Transcriptional control signals of a eukaryotic protein-coding gene. Science. 1982 Jul 23;217(4557):316–324. doi: 10.1126/science.6283634. [DOI] [PubMed] [Google Scholar]
- McLachlan A. D. Coiled coil formation and sequence regularities in the helical regions of alpha-keratin. J Mol Biol. 1978 Sep 5;124(1):297–304. doi: 10.1016/0022-2836(78)90163-8. [DOI] [PubMed] [Google Scholar]
- McLachlan A. D., Stewart M. Tropomyosin coiled-coil interactions: evidence for an unstaggered structure. J Mol Biol. 1975 Oct 25;98(2):293–304. doi: 10.1016/s0022-2836(75)80119-7. [DOI] [PubMed] [Google Scholar]
- Moll R., Franke W. W., Schiller D. L., Geiger B., Krepler R. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell. 1982 Nov;31(1):11–24. doi: 10.1016/0092-8674(82)90400-7. [DOI] [PubMed] [Google Scholar]
- Nelson W. G., Sun T. T. The 50- and 58-kdalton keratin classes as molecular markers for stratified squamous epithelia: cell culture studies. J Cell Biol. 1983 Jul;97(1):244–251. doi: 10.1083/jcb.97.1.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshima R. G., Trevor K., Shevinsky L. H., Ryder O. A., Ceceña G. Identification of the gene coding for the Endo B murine cytokeratin and its methylated, stable inactive state in mouse nonepithelial cells. Genes Dev. 1988 May;2(5):505–516. doi: 10.1101/gad.2.5.505. [DOI] [PubMed] [Google Scholar]
- Parker B. A., Stark G. R. Regulation of simian virus 40 transcription: sensitive analysis of the RNA species present early in infections by virus or viral DNA. J Virol. 1979 Aug;31(2):360–369. doi: 10.1128/jvi.31.2.360-369.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry D. A., Steven A. C., Steinert P. M. The coiled-coil molecules of intermediate filaments consist of two parallel chains in exact axial register. Biochem Biophys Res Commun. 1985 Mar 29;127(3):1012–1018. doi: 10.1016/s0006-291x(85)80045-0. [DOI] [PubMed] [Google Scholar]
- RayChaudhury A., Marchuk D., Lindhurst M., Fuchs E. Three tightly linked genes encoding human type I keratins: conservation of sequence in the 5'-untranslated leader and 5'-upstream regions of coexpressed keratin genes. Mol Cell Biol. 1986 Feb;6(2):539–548. doi: 10.1128/mcb.6.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieger M., Jorcano J. L., Franke W. W. Complete sequence of a bovine type I cytokeratin gene: conserved and variable intron positions in genes of polypeptides of the same cytokeratin subfamily. EMBO J. 1985 Sep;4(9):2261–2267. doi: 10.1002/j.1460-2075.1985.tb03924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg M., RayChaudhury A., Shows T. B., Le Beau M. M., Fuchs E. A group of type I keratin genes on human chromosome 17: characterization and expression. Mol Cell Biol. 1988 Feb;8(2):722–736. doi: 10.1128/mcb.8.2.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savtchenko E. S., Freedberg I. M., Choi I. Y., Blumenberg M. Inactivation of human keratin genes: the spectrum of mutations in the sequence of an acidic keratin pseudogene. Mol Biol Evol. 1988 Jan;5(1):97–108. doi: 10.1093/oxfordjournals.molbev.a040473. [DOI] [PubMed] [Google Scholar]
- Schermer A., Galvin S., Sun T. T. Differentiation-related expression of a major 64K corneal keratin in vivo and in culture suggests limbal location of corneal epithelial stem cells. J Cell Biol. 1986 Jul;103(1):49–62. doi: 10.1083/jcb.103.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Steinert P. M., Idler W. W., Zimmerman S. B. Self-assembly of bovine epidermal keratin filaments in vitro. J Mol Biol. 1976 Dec 15;108(3):547–567. doi: 10.1016/s0022-2836(76)80136-2. [DOI] [PubMed] [Google Scholar]
- Steinert P. M., Steven A. C., Roop D. R. The molecular biology of intermediate filaments. Cell. 1985 Sep;42(2):411–420. doi: 10.1016/0092-8674(85)90098-4. [DOI] [PubMed] [Google Scholar]
- Stoler A., Kopan R., Duvic M., Fuchs E. Use of monospecific antisera and cRNA probes to localize the major changes in keratin expression during normal and abnormal epidermal differentiation. J Cell Biol. 1988 Aug;107(2):427–446. doi: 10.1083/jcb.107.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun T. T., Green H. Keratin filaments of cultured human epidermal cells. Formation of intermolecular disulfide bonds during terminal differentiation. J Biol Chem. 1978 Mar 25;253(6):2053–2060. [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyner A. L., Eichman M. J., Fuchs E. The sequence of a type II keratin gene expressed in human skin: conservation of structure among all intermediate filament genes. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4683–4687. doi: 10.1073/pnas.82.14.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyner A. L., Fuchs E. Evidence for posttranscriptional regulation of the keratins expressed during hyperproliferation and malignant transformation in human epidermis. J Cell Biol. 1986 Nov;103(5):1945–1955. doi: 10.1083/jcb.103.5.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viac J., Staquet M. J., Thivolet J., Goujon C. Experimental production of antibodies against stratum corneum keratin polypeptides. Arch Dermatol Res. 1980;267(2):179–188. doi: 10.1007/BF00569104. [DOI] [PubMed] [Google Scholar]
- Weiss R. A., Eichner R., Sun T. T. Monoclonal antibody analysis of keratin expression in epidermal diseases: a 48- and 56-kdalton keratin as molecular markers for hyperproliferative keratinocytes. J Cell Biol. 1984 Apr;98(4):1397–1406. doi: 10.1083/jcb.98.4.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y. J., Parker L. M., Binder N. E., Beckett M. A., Sinard J. H., Griffiths C. T., Rheinwald J. G. The mesothelial keratins: a new family of cytoskeletal proteins identified in cultured mesothelial cells and nonkeratinizing epithelia. Cell. 1982 Dec;31(3 Pt 2):693–703. doi: 10.1016/0092-8674(82)90324-5. [DOI] [PubMed] [Google Scholar]