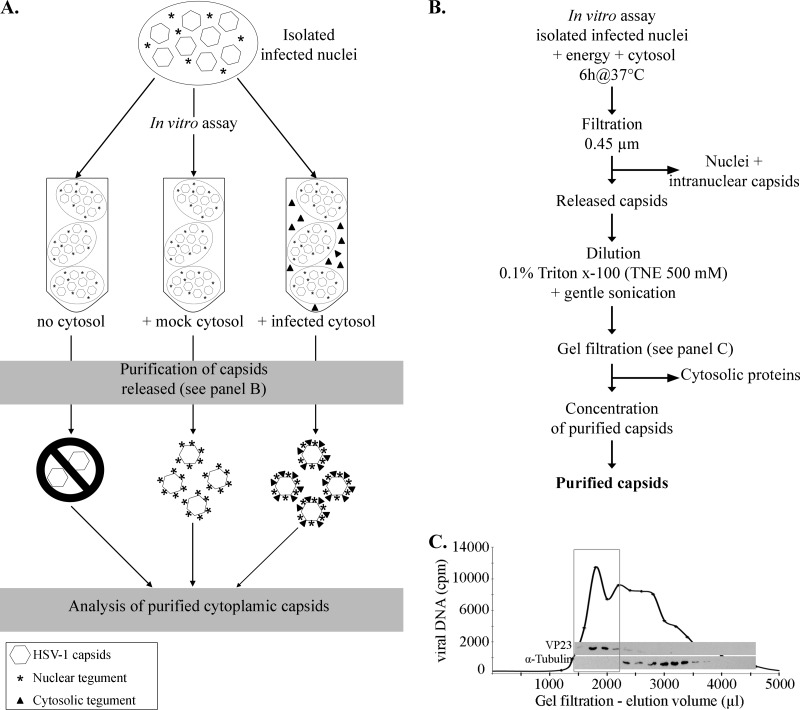
Fig 1.
Purification protocol. (A) Schematic illustration of the in vitro assay and expected results. In this scheme, infected nuclei were incubated in vitro in the presence of energy, nuclear buffer, and one of three conditions: no cytosol, cytosol derived from uninfected cells (mock-infected cytosol), or cytosol prepared from HSV-1-infected cells (infected cytosol). Note that in the absence of cytosol, very few capsids should be produced. (B) Depiction of the protocol used to purify HSV-1 cytoplasmic capsids produced in the in vitro assay. See Materials and Methods for details. (C) Enrichment of the in vitro capsids by gel filtration. The viral DNA was radiolabeled by the addition of [3H]thymidine to infected cells, and the nuclei were isolated as detailed in Materials and Methods. After their incubation in vitro with uninfected cytosol and preliminary steps of purification (as described for panel B), the cytoplasmic capsids were purified by gel filtration and fractions collected. An equivalent volume from each fraction was analyzed for its radioactivity level to detect viral DNA and probed by Western blotting against the capsid protein VP23 and against α-tubulin, a host protein. The box denotes fractions enriched in viral DNA and capsid proteins, which were subsequently pooled and concentrated before proceeding to further analysis.