Fig 2.
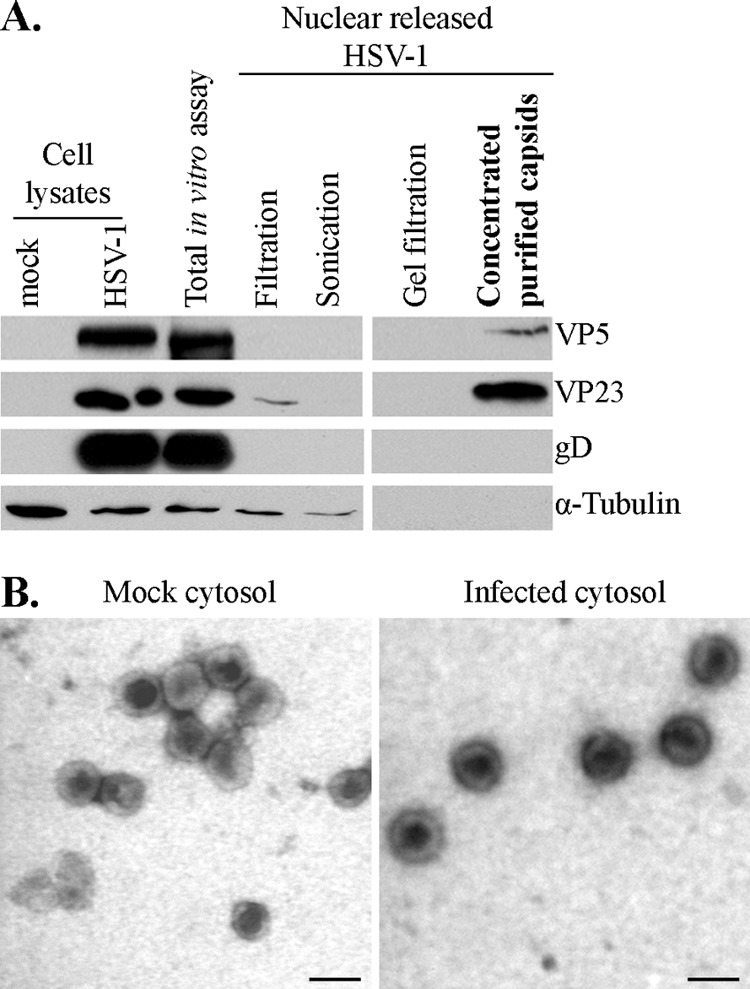
Purity of in vitro-released HSV-1 capsids. (A) Two-microgram aliquots from each step of the purification method were analyzed by SDS-PAGE and Western blotting to evaluate the purity of the samples following the in vitro assay done in the presence of mock-infected cytosol. The final step (concentrated purified capsids) was enriched in capsid proteins (VP5 and VP23) and devoid of gD, a glycoprotein associated with the viral envelope. Alpha-tubulin, a cellular component, was also absent from the final enriched material. (B) Capsids purified as described for panel A were subsequently visualized by negative staining and EM to visually inspect their purity. (Left) Capsids released from nuclei incubated in mock-infected cytosol. (Right) Capsids released from nuclei incubated in infected cytosol. Scale bar, 100 nm.
