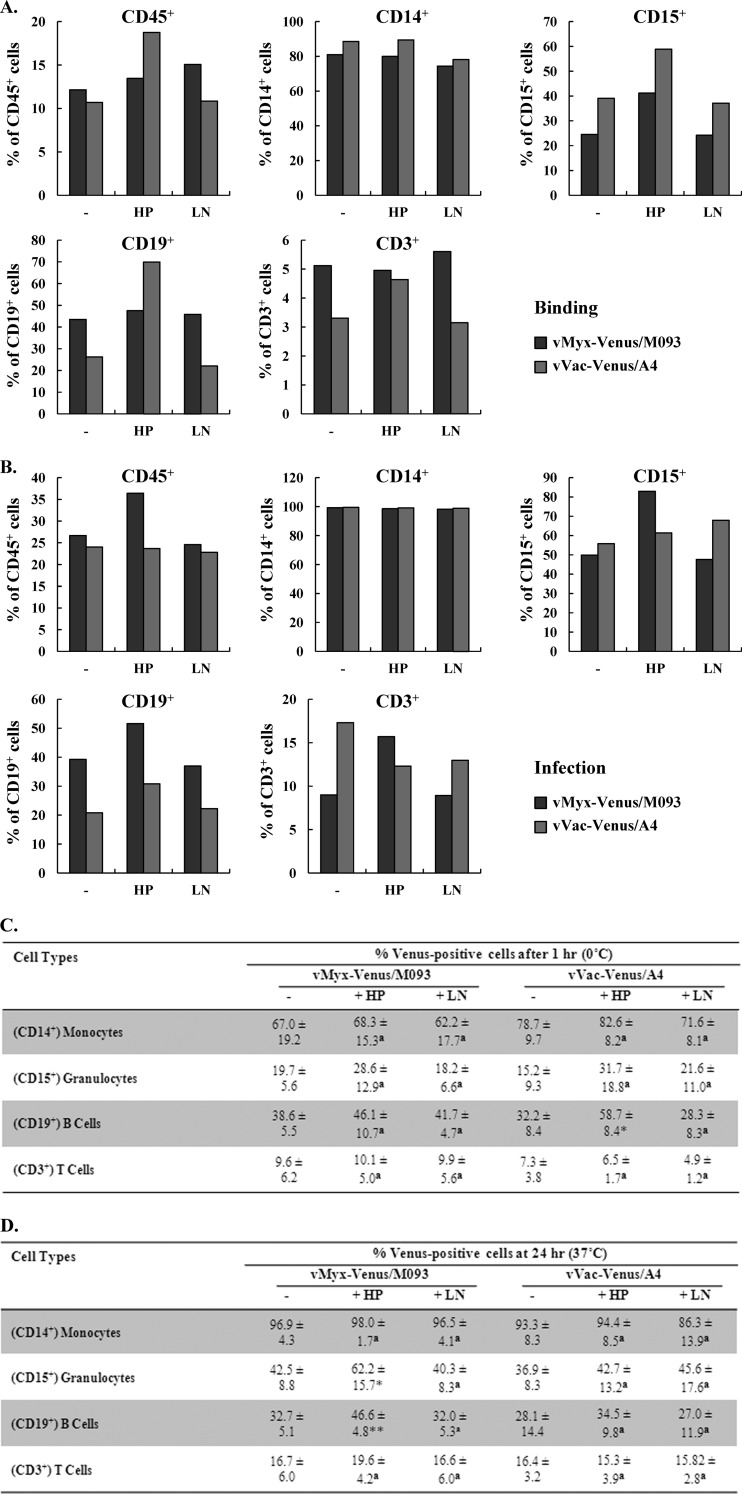
Fig 7.
Binding of Venus-tagged MYXV or VACV to primary human leukocytes is not mediated by either cell surface heparan sulfate or the extracellular matrix protein laminin. Buffy coat preparations of fresh primary human leukocytes from healthy donors were incubated with mock-, HP-, or LN-treated vMyx-Venus/M093 or vVac-Venus/A4 at an MOI of 10.0 on ice for 1 h. (A) For binding, cells were washed after incubation with virus on ice. (B) For infection, cells were further incubated at 37°C for 24 h after virion adsorption. After binding or infection, cells were stained with phycoerythrin-conjugated anti-CD45 and allophycocyanin-conjugated anti-CD14, anti-CD15, anti-CD19, or anti-CD3 lineage markers. The percentage of Venus-positive cells within each population was determined by flow cytometry. A representative of four independent experiments is shown. (C and D) Summary of the types of primary human cells from donor PBMCs susceptible to MYXV or VACV binding and infection. Average percentages of different cell populations bound (C) or infected (D) by vMyx-Venus/M093 or vVac-Venus/A4 after 1 h at 0°C or 24 h at 37°C, respectively, as detected by flow cytometry under various treatments, were determined. Averages of four independent samples and standard deviations are shown. Statistical analyses between the untreated and HP- or LN-treated groups for each virus were performed using the Student t test. * and **, P ≤ 0.05 and P ≤ 0.005, respectively, which are considered significant; a, P > 0.05, which is considered insignificant.