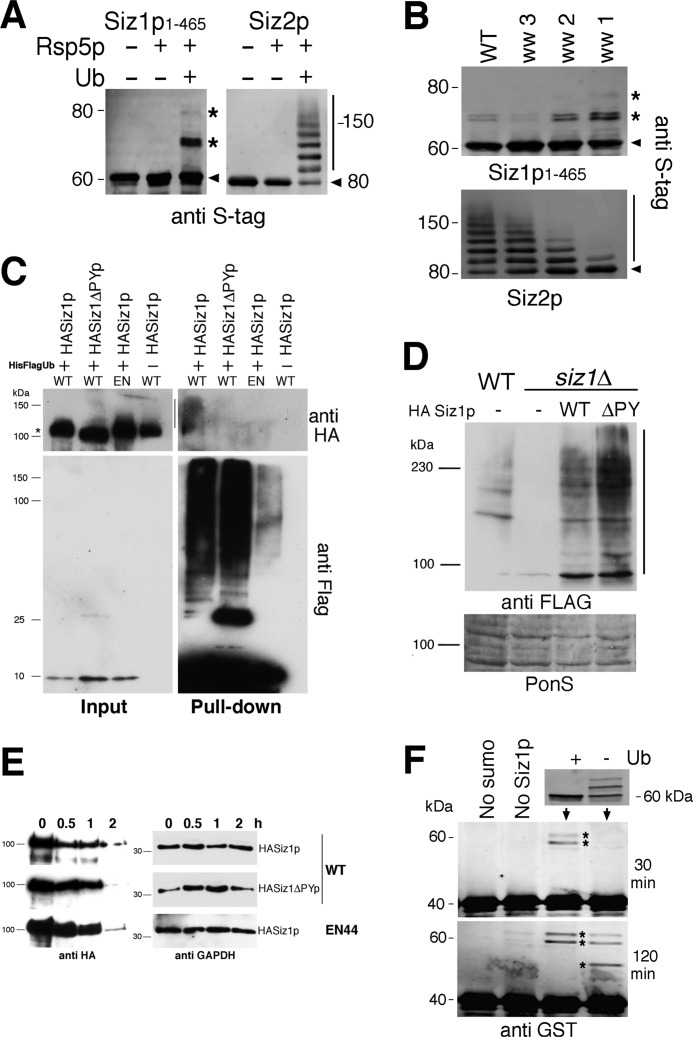
FIGURE 4.
A, in vitro ubiquitylation assays with recombinant Rsp5p and Siz1p(1–465) and Siz2p (10% SDS-PAGE). Arrowheads indicate unmodified Siz proteins; asterisks and bars indicate major modified forms of Siz1p(1–465) and Siz2, respectively. B, in vitro ubiquitylation of recombinant Siz1p(1–465) and Siz2p with either wild-type (WT) Rsp5p or variants in which only the indicated WW domain remains functional. Asterisks and bars indicate major modified forms of Siz1p(1–465) and Siz2, respectively. C, co-purification under denaturing conditions of His-FLAG-tagged ubiquitin with HASiz1p. Wild-type and an EN44 strain (EN) were co-transformed with HASiz1p or a version that lacks PY motifs (HASiz1ΔPYp) along with His-FLAG-tagged ubiquitin. Cells were grown to log phase, and cell lysates were prepared under denaturing conditions using a 6 m guanidine HCl buffer and pulldown assays carried out using His-select resin. Immunoblotting was performed using anti-HA (upper panels) and anti-FLAG antibodies (lower panels) on input (left panels) and pulldown (right panels) samples. Asterisk indicates position of HASiz1p, and bar shows position of modified HASiz1p. D, upper panel, total cell lysates expressing FLAG-Smt3p in a wild type yeast (WT) or siz1Δ strain were immunoblotted using anti-FLAG antibodies alongside total cell extracts from cells co-expressing an empty FLAG vector (−), HA-Siz1p (WT), or HA-Siz1pΔPY (ΔPY) as indicated. Lower panel, protein loading as assessed by Ponceau S staining (PonS). E, cycloheximide chase experiments with WT and an EN44 strain expressing HASiz1p or a version that lacks PY motifs (HASiz1ΔPYp). Cells were grown to log phase, and samples were taken at the time intervals indicated following the addition of 100 μg ml−1 cycloheximide. Immunoblotting was performed on the same samples using either anti-HA or anti-GAPDH antibodies. F, upper panel, recombinant Siz1p(1–465) (as visualized using anti-S-tag antibodies on 12% SDS-PAGE) subjected to in vitro ubiquitylation reaction with Rsp5p (+) with a negative control that contained all the ubiquitylation components except ubiquitin (−). Lower panels, modified and unmodified Siz1p(1–465) used in an in vitro SUMOylation reaction performed for 30 and 120 min using GST-RanGap1p(241–360) as a substrate. Asterisks indicate positions of SUMOylated GST-RanGap1p(241–360).