FIGURE 5.
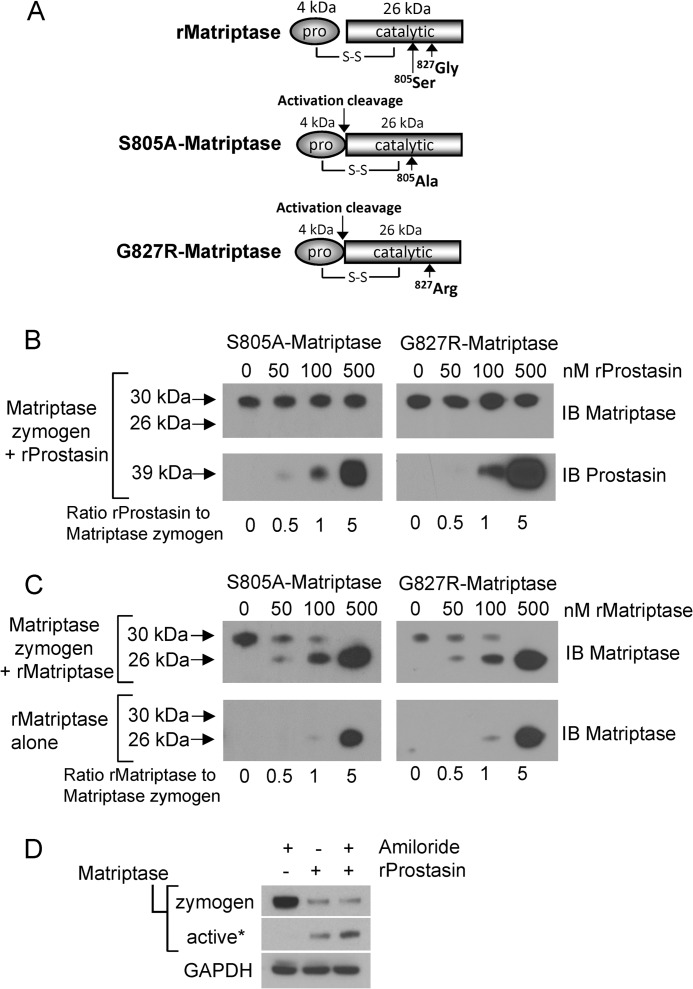
Prostasin is not capable of direct activation cleavage of the matriptase zymogen. A, schematic representing the recombinant wild type and mutant matriptase serine protease domains. Matriptase is capable of intermolecular autoactivation by proteolytic cleavage between the pro- and catalytic domains, and recombinant matriptase (rMatriptase) possesses full catalytic activity. It is resolved under reducing conditions as a ∼26-kDa protein that is detected using an antibody generated against the serine protease domain. The matriptase mutants S805A-matriptase and G827R-matriptase are single chain zymogens that under reducing conditions retain the 4-kDa prodomain and are resolved at ∼30 kDa. S-S represents the disulfide link between the pro- and catalytic domains. B, the matriptase zymogen is not a prostasin substrate. 100 nm S805A-matriptase or G827R-matriptase were incubated with increasing concentrations of recombinant prostasin (50, 100, 500 nm) for 1 h at 37 °C. Reactions were immunoblotted (IB) using anti-matriptase antibodies. No change in the S805A-matriptase or G827R-matriptase molecular weight is observed, indicating lack of proteolytic cleavage. The blot was reprobed for prostasin to show its presence in the reactions (B, lower panels). C, the matriptase zymogen is a matriptase substrate. 100 nm S805A-matriptase or G827R-matriptase were incubated with increasing concentrations of recombinant matriptase (50, 100, 500 nm) for 1 h at 37 °C. Reactions were immunoblotted (IB) using anti-matriptase antibodies. Loss of the 30-kDa zymogen forms are observed and are accompanied by appearance of the 26-kDa catalytic domain. The lower immunoblot is of a control assay to show the amounts of recombinant matriptase added to the reaction mix because the added recombinant matriptase overlays the released 26kDa catalytic domain. D, amiloride does not inhibit prostasin-induced matriptase cleavage and activation. Immunoblots of cell lysates prepared at 4 h show that activation of matriptase by recombinant prostasin is not inhibited by the presence of amiloride. An asterisk indicates that the blot was overexposed to detect the liberated serine protease domain as a measure of active matriptase.