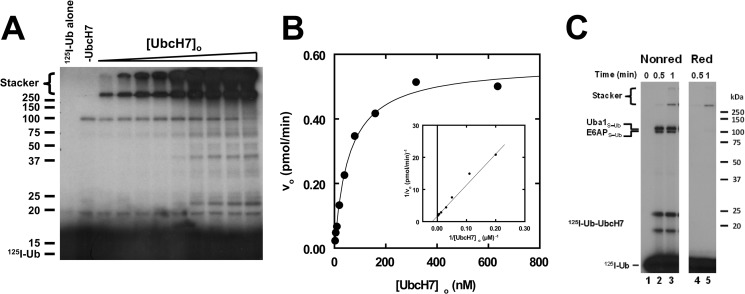
FIGURE 2.
UbcH7 exhibits hyperbolic kinetics for E6AP-catalyzed polyubiquitin chain formation. A, shown is a representative autoradiogram of E6AP-catalyzed polyubiquitin chain formation in which 125I-ubiquitin conjugation assays containing 30 nm GST-E6AP were conducted under initial velocity conditions in the absence (−UbcH7) or presence of 2–650 nm UbcH7 as described under “Materials and Methods.” After autoradiography, 125I-ubiquitin conjugates larger than 25 kDa were excised and quantified by γ-counting, then the absolute rate was calculated using the specific radioactivity of the labeled ubiquitin (42, 43). B, shown is the concentration dependence of initial velocity versus [UbcH7]o from the assay shown in panel A. The solid line represents the nonlinear hyperbolic regression fit of the data for kinetic constants listed in Table 1. The inset shows the double reciprocal plot of the data. C, shown is the time course for 125I-ubiquitin thioester formation resolved by non-reducing (Nonred) and reducing (Red) conditions as described under “Materials and Methods” for assays containing 38 nm Uba1, 128 nm UbcH7, and 12 nm E6AP. Mobility of molecular weight markers (kDa) are shown to the left (panel A) or right (panel C). For panel C, positions of the various thioester are shown to the left. The UbcH7-125I-ubiquitin thioester migrates as two bands due to an artifact of incomplete denaturation under non-reducing conditions.