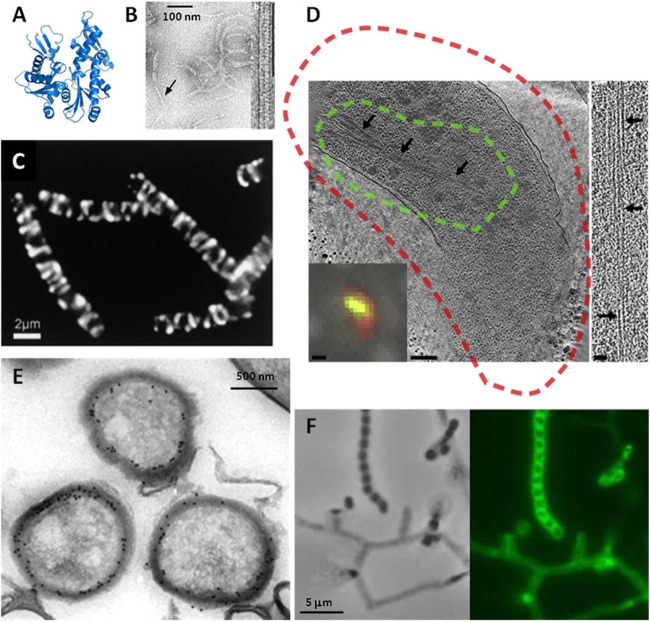
Fig 2.
Structure and microscopy of MreB. (A and B) The fold of prokaryotic MreB is similar to that its eukaryotic counterpart, actin (A), and MreB assembles into actin-like filaments (B). (C) The seemingly helical localization of an (MreB-like) Mbl-GFP fusion protein in cells in rod-shaped B. subtilis is now called into question (41–43). (D) In fact, though cytoplasmic MreB filaments are visible in tomograms, no helical filaments were seen in several rod-shaped bacteria, and helical filaments seen in E. coli were shown to be an artifact of the N-terminal YFP tag. In panel D, a tomographic slice through a Vibrio cholerae cell overexpressing GFP-MreB is shown. The cryo-fLM inset shows the cell stained with membrane dye FM 4-64 (red) and expressing GFP-MreB (green); dashed lines in the main image represent the fluorescent signal boundaries. On the right side, a 15-nm-thick tomographic slice through an MreB bundle not fused to GFP is shown. The scale bars represent 1 μm in the fLM inset, 200 nm in the cryo-electron tomography slice, and 50 nm in the higher-magnification inset. In Streptomyces coelicolor, MreB is not essential for vegetative growth but is essential for spore integrity and has been shown by immunoelectron microscopy (E) and fluorescence microscopy (F) to localize to the spore envelope. Panel B adapted from reference 33 with permission from the Nature Publishing Group; panel C from reference 14 with permission from Elsevier Ltd.; panel D from reference 42 with permission from Elsevier Ltd.; and panels E and F from reference 59 with permission from John Wiley and Sons.