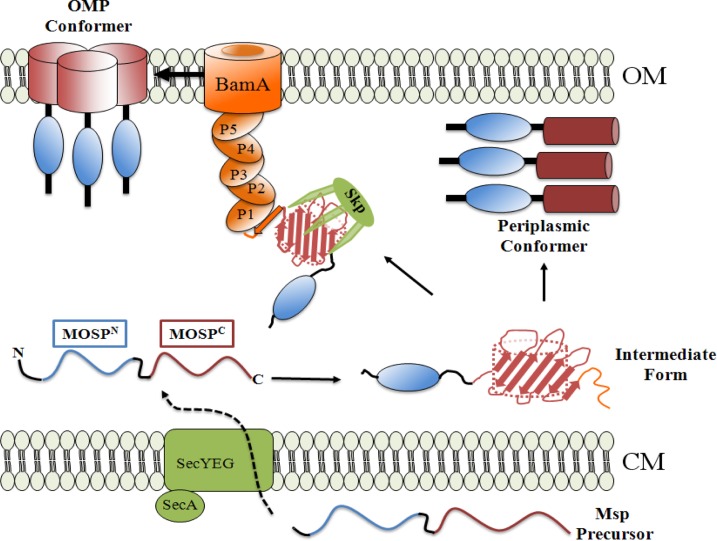
Fig 9.
Proposed two-pathway model for generation of amphiphilic, OM-inserted and soluble, periplasmic Msp conformers. Msp precursor is exported across the cytoplasmic membrane (CM) by the Sec translocon. Once within the periplasm, the unfolded precursor acquires some secondary structure, becoming the “intermediate form.” The intermediate form can either be chaperoned via Skp (Tde2602) to the POTRA arm of BamA (Tde2601) for OM assembly (25) and trimerization (OMP conformer) or fold into a soluble trimeric periplasmic conformer. We hypothesize that in the intermediate form, a stretch of amino acids at the extreme C terminus of MOSPC (i.e., the signature sequence, shown in orange) containing the BamA recognition signal is unfolded but can become a β-strand once in contact with the POTRA1 domain of BamA. The resultant block to entry into the OMP assembly pathway drives the intermediate form toward the alternative, periplasmic pathway. The OMP conformer is depicted as trimerizing via the MOSPC domains based on our prior analysis of TprC (40). Presently, we have no data as to the regions of Msp that mediate trimerization of the periplasmic conformer. The components of the Bam complex in T. denticola, other than BamA, are unidentified (25) and, therefore, not shown.