Fig 4.
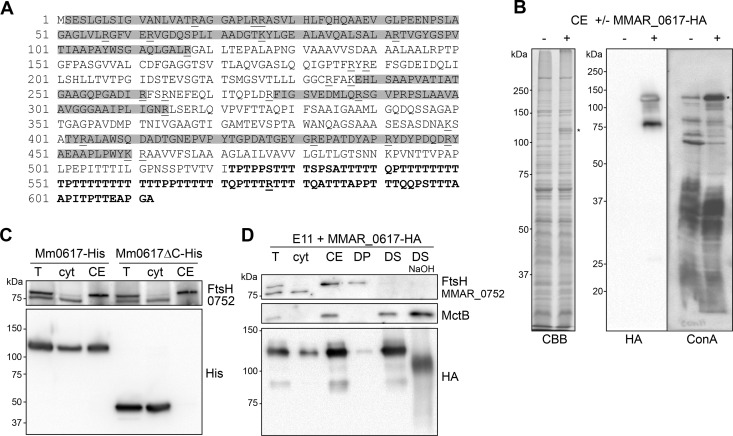
Extensive modification of MMAR_0617. (A) Amino acid sequence of MMAR_0617. Highlighted in gray are peptide sequences that were detected by MS/MS analysis and therefore do not contain modifications. Underlined are trypsin digestion sites, and the Thr-rich C-terminal domain that is deleted in the truncated MMAR_0617ΔC is indicated in bold. (B) CBB staining and immunoblots using the HA antibody and horseradish peroxidase (HRPO)-conjugated ConA of cell envelope fractions (CE) without (−) and with (+) MMAR_0617-HA (indicated with *). (C) Immunoblot of total lysate (T), cytosol (cyt), and CE of M. marinum expressing MMAR_0617-His or MMAR_0617ΔC-His loaded in a 1:1:1 ratio. (D) Immunoblot of subcellular fractions of M. marinum expressing MMAR_0617-HA probed with anti-FtsH, anti-MctB, and anti-HA antibodies; T, cyt, CE, detergent pellet (DP), and supernatant (DS) obtained after solubilization of CE with 1% OBG and DS treated with 0.15 M NaOH (DS NaOH) loaded in a 1:1:3:5:5:5 ratio.