Abstract
Study Objectives:
To develop a method, called Caenorhabditis-in-Drop (CiD), encapsulating single worms in aqueous drops, for parallel analysis of behavioral quiescence in C. elegans nematodes.
Design:
We designed, constructed, and tested a device that houses an array of aqueous droplets laden with individual worms. The droplets are separated and covered by immiscible, biocompatible oil. We modeled gas exchange across the aqueous/oil interface and tested the viability of the encapsulated animals. We studied the behavior of wild-type animals; of animals with a loss of function mutation in the cGMP-dependent protein kinase gene egl-4; of animals with a loss of function mutation in the gene kin-2, which encodes a cAMP-dependent protein kinase A regulatory subunit; of animals with a gain-of-function mutation in the gene acy-1, which encodes an adenylate cyclase; and of animals that express high levels of the EGF protein encoded by lin-3.
Measurements and Results:
We used CiD to simultaneously monitor the behavior of 24 worms, a nearly 5-fold improvement over the prior best methodology. In support of our gas exchange models, we found that worms remain viable on the chip for 4 days, past the 12-h period needed for observation, but show reduced longevity to that measured on an agar surface. Measurements of duration of lethargus quiescence and total leth-argus quiescence showed reduced amounts as well as reduced variability relative to prior methods. There was reduced lethargus quiescence in animals that were mutant for kin-2 and for acy-1, supporting a wake-promoting effect of PKA in C. elegans, but no change in lethargus quiescence in egl-4 mutants. There was increased quiescence in animals that expressed kin-2 in the nervous system or over-expressed EGF.
Conclusions:
CiD is useful for the analysis of behavioral quiescence during lethargus as well as during the adult stage C. elegans. The method is expandable to parallel simultaneous monitoring of hundreds of animals and for other studies of long-term behavior. Using this method, we were successful in measuring, for the first time, quiescence in kin-2(ce179) and in acy-2(ce2) mutants, which are hyperactive. Our observations also highlight the impact of environmental conditions on quiescent behavior and show that longevity is reduced in CiD in comparison to agar surfaces.
Citation:
Belfer SJ; Chuang HS; Freedman BL; Yuan J; Norton M; Bau HH; Raizen DM. Caenorhabditis-in-drop array for monitoring C. elegans quiescent behavior. SLEEP 2013;36(5):689-698.
Keywords: General, drop, elegans, lethargus, microfluidics, quiescence
INTRODUCTION
The nematode Caenorhabditis elegans is a powerful genetic model animal in the study of behavior. To date, most studies of C. elegans have focused on the analysis of a behavior on short time scales, ranging from milliseconds to several minutes. More recently, efforts have been made toward understanding C. elegans behavior on longer time scales, ranging from hours to days. Many of the current automated imaging techniques are optimized for short term observations and cannot be easily adapted to study long-term behavior.
A behavior that happens on the scale of hours occurs during lethargus, a sleep-like quiescent behavioral state that precedes larval molts in C. elegans.1 Similar to sleep in other organisms, lethargus is characterized by cessation of feeding and motion, reduced responsiveness to external stimuli, and homeostatic regulation.2 The demonstration that genetic regulators of mammalian and Drosophila sleep also regulate lethargus behavior2–4 supports the notion that lethargus behavior and sleep share a common evolutionary origin. Therefore, using C. elegans to discover and study novel genetic regulators of lethargus behavior will improve our understanding of sleep regulation in other animals including humans.
Initial approaches for measuring lethargus behavior relied on monitoring worms on an agar surface seeded with bacteria.2,4 However, this method is limited to single-worm recordings, and some worms do not stay confined in the field of the camera view for the duration needed for the recording. Two recent solutions have been proposed, both making use of microfluidics technology. In the first, Singh et al.5 devised a microfluidics chip composed of 6 chambers, each with the texture of artificial dirt.6 The depth of these chambers was optimized for fourth larval stage (L4) animals. In a second approach, Bringmann devised aga-rose hydrogel compartments to constrain individual first larval stage (L1) animals for high-magnification fluorescent imaging of L1 animals.7 While an improvement over single-worm imaging, both of these methods remain relatively low throughput and are suitable for the imaging of only one lethargus stage.
To increase the throughput of assessment of lethargus behavior, we here describe a drop-based technology originating from the concept of lab-in-a-drop.8 Aqueous droplet analysis has been proven to be a powerful tool in many biochemical applications.9,10 This approach eliminates the need for the structural valves, pumps, and channels used in many other microfluidic designs. Additionally, a droplet-based platform enhances the freedom of motility of the worms and simplifies the production of the chips and experimental preparation. In a similar concept to ours, Shi et al. showed the ability to encapsulate worms in droplets and position them in an array on a microfluidic chip. They noted that surrounding the aqueous droplet laden with a worm with a biocompatible gas respiratory oil allows sufficient gas exchange for organisms to survive over long periods.11
In our study, we used sub-microliter droplets containing E. coli bacteria as a food source to encapsulate single worms for long-term observations. This method can support worms for several days, enabling imaging throughout lethargus. The small volume of each droplet allows for the preparation of a dense array of droplets, thereby permitting the simultaneous imaging of at least 24 worms while retaining the identity of individual worms. We find a high degree of reproducibility among individual animals, allowing for differentiation of wild-type worms from lethargus behavioral mutants. With the simplicity of this system, the Caenorhabditis-in-Drop technique allows for high throughput analysis of complex behaviors.
METHODS
PDMS Chip Preparation
Micro wells were formed by casting polydimethylsiloxane elastomer (PDMS, Sylgar 184, Ellisworth Adhesives) on a mold made with photoresist (SU-8 2025, Microchem). Each well in the 4×6 array had a diameter of 1 mm and a depth of 65 μm. The pitch of the array was 1.2 mm. Immediately before use, an individual chip was treated with oxygen plasma (Diener, Femto Standard) to increase the surface energy of the PDMS and render it hydrophilic, cleaned by lifting dirt particles with Scotch tape, and placed in a 3-centimeter diameter Petri dish. Distilled water was included in the dish to surround the PDMS chip and thus to increase the humidity of the recording chamber.
Preparation and Loading of Worms into Droplets
A suspension of HB101 E. coli bacteria12 was cultured overnight in LB broth at 37°C. One mL of the stationary phase culture was concentrated by centrifugation, followed by resus-pension in 67 microliters of liquid NGM. Absorbance at 600 nm (Nanodrop 2000, Thermo Scientific) of the agitated concentrated bacterial suspension13 varied from 0.75 to 1.0. The bacteria suspension was used to form the sub-microliter drops.
Worms were cultured and handled as previously described,14 cultivated at 20°C on NGM agar plates and fed the streptomycin-resistant E. coli strain HB101.12 The wild-type strain used was N2.14 Other strains used were KG532 [kin-2(ce179) X],15 MT1074 [egl-4(n479) IV],16 FK234 [egl-4(ks62) IV],17 and KG518 [acy-1(ce2) III],15 PS5009 [pha-1(e2132ts) III; him-5(e1490) V; hs::LIN-3C; Pmyo-2:GFP; pha-1(+)]4; NQ203 [kin-2(ce179) X; Punc-119:kin-2], and NQ226 [kin-2(ce179) X; Pdpy-7:kin-2]. L4 stage worms were selected based on size and the developmental stage of the vulva observed under 25x total magnification using a Leica MS5 stereomicroscope.
An aqueous drop of NGM with concentrated HB101 was added using a p2 Pipetman (Gilson) into each well until filled. A single loading of 2 microliters was sufficient to fill 16 wells. Thus, we estimate the volume of individual wells to be approximately 125 nL. After filling the wells with the bacterial suspension, the drops were covered with an air-permeable, biocompatible heavy lab grade mineral oil (Fisher Scientific Catalog number S66138), which prevented evaporation and coalescing of the droplets. L4 stage worms were then transferred without bacteria through the mineral oil layer and into the individual droplets using a platinum wire pick covered with a film of mineral oil. A cross-section of the array of droplets laden with worms is depicted schematically in Figure 1A. Figure 1B is a top view photograph of the array.
Figure 1.
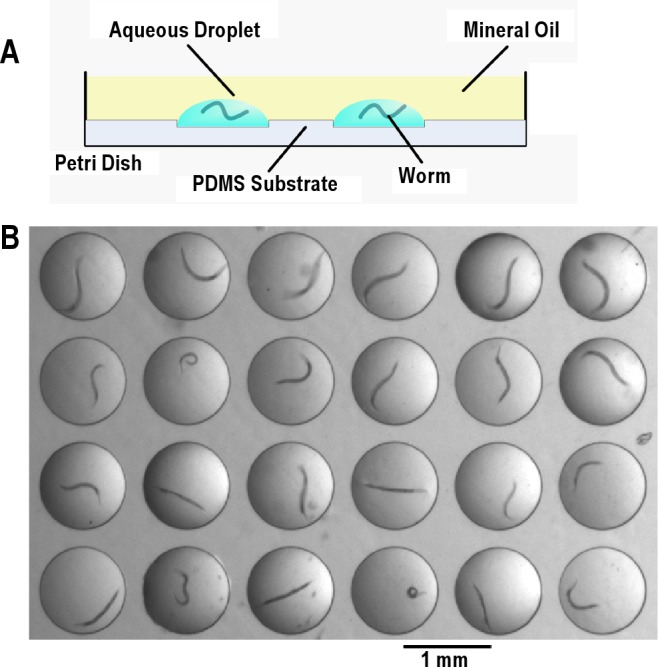
Experimental set up. (A) Cross-section schematic of microfluidic chip. (B) Top view of the image of 24 worms. Shown is a picture of 24 L4 worms, each in an individual droplet on a microfluidic chip. The mineral oil on top of the droplets is transparent.
The Petri dish housing the PDMS chip containing the loaded worms and immersed in mineral oil was then covered with a transparent dish cap to minimize potential disturbances by ambient airflow. The array of worms in drops was placed on a Diagnostics Instruments microscope base and illuminated for bright field microscopy, using white light supplied to the base with a fiberoptic cable from a Schott DCR III light source. A Zeiss Stemi 2000 stereomicroscope under 0.65-0.80 objective magnification was used to visualize the worms in the 4x6 array of droplets. At this magnification and our camera acquisition setting, the spatial resolution was 7.2 micrometers per pixel. A camera (659x494 pixels, scA640-70fm, Basler vision technologies) was mounted on the microscope and set to acquire images with 8-bit grayscale resolution at 1-sec intervals. Experiments were performed in a constant temperature environmental room set to 20 ± 1°C. In 2 experiments (comparing FK234 to N2 behavior and comparing NQ203 to KG532 behavior), worms of the 2 genotypes were monitored simultaneously but in different wells of the same CiD chip.
Longevity Experiments
Longevity experiments were carried out at 20°C. Longevity was assessed of worms housed individually in the following arenas: 125 nL aqueous droplets covered with mineral oil on a PDMS surface; 1,250 nL aqueous droplets covered with mineral oil on a PDMS surface; NGM agar surface seeded with an annulus (inner diameter ~1 cm, outer diameter ~3 cm) of HB101 bacteria that had been grown for 12 h at 37° in LB medium. The HB101 bacteria seeded on the agar surface was allowed to dry at room temperature for 24-48 h prior to use. The concentration of HB101 bacteria in the solutions housing the worms was adjusted such that the optical density at 600 nm ranged from 0.75 to 1.00. FUdR at a concentration of 100 micrograms/mL was added to the liquid housing the worms to prevent progeny production. FUdR was used only for longevity assessments and was not included for routine measurements of L4 lethargus quiescence. The worms were introduced into the CiD device as mid L4s after growing on an agar surface seeded with bacteria from hatching. Every 24 h in the CiD device, the worms were visually inspected for spontaneous movements. If a worm did not move spontaneously or in response to gentle tapping, it was stimulated with platinum wire inserted through the oil. Worms that did not respond to 3 such stimulations were regarded as dead, as is commonly considered in C. elegans aging research. Animals that were injured during transfer and animals on an agar surface that crawled on the walls of the Petri dish were censored from the analysis. The experiments were performed in duplicate or triplicate.
Image Processing
To determine whether the worm was quiescent or active, we used the frame-subtraction algorithm developed by Raizen et al.2,18 Briefly, two 8-bit gray scale (GS) digital images taken τ seconds apart, were subtracted using the formula ΔGS(XiYj) = (GS2(XiYj) − GS1(XiYj)) / 2 + 128, where ΔGS(XiYj) is the difference grayscale value centered at 128 at X position i and Y position j. As done previously, in each experiment, we performed the subtraction analysis on a region in the array that did not contain a worm in order to determine the magnitude of the noise in the experiment. During the behavior monitoring, when ΔGS at every pixel was less than the noise range, the animal was assigned an instantaneous quiescent value of one. Otherwise, it was considered to have moved between the pair of subtracted images and was assigned an instantaneous quiescent value of zero. Accordingly, the analysis of the video frames yielded a binary time series of ones and zeroes for each animal in the array. The time series was then averaged over a 10-min moving time interval to yield the quiescence fraction in that interval. We defined the start of lethargus quiescence as the time at which the fraction of quiescence rose to above a predetermined threshold and then remained above this threshold for ≥ 20 minutes. The thresholds used were, respectively, 0.2, 0.15, 0.1, 0.05, and 0.05 for time series of subtraction intervals τ = 1-second, 2-second, 5-second, 8-second, and 10-second. Likewise, we defined the end of lethargus quiescence as the time at which the fraction of quiescence dropped below the threshold and remained below that level ≥ 20 minutes. Worms that had already started their lethargus period prior to the beginning of the recording or were injured during transport were censored from the analysis. The number of worms censored for this reason ranged from 0-6 worms per 24 worms in a single-chip experiment.
For analysis of quiescence of kin-2;Punc-119:kin-2 transgenic animals and of lin-3 over-expressing animals, in which extensive quiescence was observed outside of lethargus (Figure S3, S4), we measured the total quiescence in the 10-h recording period and compared this to total quiescence of control animals in the same recording period. Because the start of lethargus was not clearly defined in these transgenic animals, we did not censor animals in this experiment.
RESULTS
We designed and fabricated a PDMS chip containing an array of miniature wells (Figure 1). We loaded the wells with buffer laden with a concentrated suspension of bacteria, covered the droplets with mineral oil to prevent water evaporation and to keep droplets from coalescing, and loaded individual worms through the oil into individual droplets (Figure 1B). The whole process of loading the chip with 24 worms lasted < 20 minutes. We will refer to the method as the Caenorhabditis-in-Drop (CiD) method.
Gas Exchange through the Oil Layer
The usefulness of the CiD system depends on the worm's ability to survive and retain nearly normal behavior while encapsulated in the oil-enveloped aqueous drop. Prior studies have shown viability for several days of organisms confined in aqueous compartments covered with oil.11,19,20 This suggests that the oils used to prevent evaporation of the aqueous phase have sufficient gas permeability to support life. To examine this hypothesis, we estimated the transport of oxygen (O2) and carbon dioxide (CO2) between the drop and the ambient air. The model is described in the supplemental information and is based on the work of Baltz and Biggers (1991). The properties of gases used in the model are listed in Table S1. Our calculations indicate that, due to the high solubility of gases in the oil, the oil layer had little effect on the O2 concentration distribution in the water drop compared to that in a drop directly exposed to air, and that the oxygen supply under our setup is sufficient for sustaining an adult worm indefinitely.
The estimated CO2 concentration (0.88 mM) is far below the CO2 concentration in air reported to be harmful to C. elegans (~3.68 mM).21 According to Khanna et al.,22 C. elegans has high tolerance to pH variations and can survive in the pH range between 3.2 and 11.8 for ≥ 96 hours. Our calculations (supplemental information) and prior literature support the feasibility of using a drop-based reactor for high throughput, long-term monitoring of C. elegans behavior.
Worm Longevity on the CiD Array
While our quiescence experiments were limited to 10 h of recording, for other purposes, it might be desirable to record worms' behavior for longer durations. For example, analysis of circadian rhythms requires several days of recording. Prior studies have suggested that worms can survive for several days while constrained in PDMS chambers.23 However these latter chips were open with each chamber equipped with an inlet and outlet, permitting flow through of a regular supply of fresh food and removal of animal waste. In contrast, our CiD design consists of a closed system, where the only food available to the worm is the food supplied in the original droplet, and where all non-gaseous animal waste products are trapped within the droplet for the duration of the experiment.
Prior experiments utilizing drop-based reactors to study C. elegans have demonstrated that survival is possible for ≥ 5 days, allowing the study of worms' response to environmental perturbations,11 but no systematic studies of worm longevity were carried out. We therefore tested the longevity of worms in our microfluidics chip. To minimize the chance of matricide and to prevent the worms' progeny from consuming the food supply, we included the DNA synthesis inhibitor FUdR24 in the droplets. All our experiments were carried out at 20°C. We found that > 80% of the worms were alive after 5 days in the droplets but that survival rapidly decreased, such that by 12 days, all worms were dead. The survival in CiD was greatly reduced in comparison to that on an agar surface (Figure 2). We found that housing the worms in a 10-fold higher volume of aqueous solution on a PDMS surface increased survival in comparison to survival in the typical volume (125 nL) used in the CiD device (Figure 2), suggesting that reduced longevity is not explained by the liquid habitat, by exposure to mineral oil, or by exposure to the PDMS surface. We propose additional explanations for reduced survival in CiD in our discussion.
Figure 2.
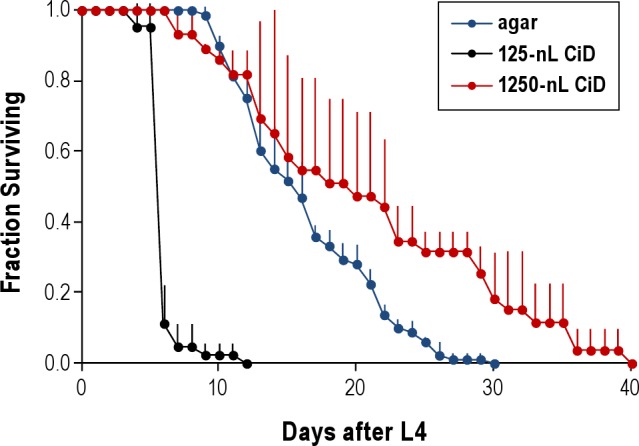
Survival of worms in the CiD device. Shown is the fraction of worms alive as a function of the day after the L4 stage. Time Zero corresponds to the middle of the L4 stage. Survival on agar was assessed on 3 separate Petri dishes, each housing 24-30 worms. Survival in 125 nL in the CiD device was assessed on 2 separate chips, each housing 21-28 worms. Survival in 1,250 nL in the CiD device was assessed in 3 separate chips, each housing 8-11 worms. Error bars denote standard deviation.
Effect of Interval of Subtraction on Quiescence Measurements
To identify epochs of behavioral quiescence, we subtracted pairs of video images taken τ seconds apart as previously described.2,18 In prior recordings of worms on an agar surface2 and in artificial dirt,5 an interval of subtraction of τ = 10 seconds was used. However, it has long been known that nematodes are far more active in liquid media than on an agar surface,25 perhaps due to reduced mechanical load in liquid26 or to a different motor program in liquid.27 It was therefore necessary to reduce the subtraction interval. The increased motion of the worms is due to deliberate movement and not due to background flow currents in the drop. We determined that convective currents in the drop do not play any significant role by suspending dead worms in the drop and observing no discernible motion (data not shown).
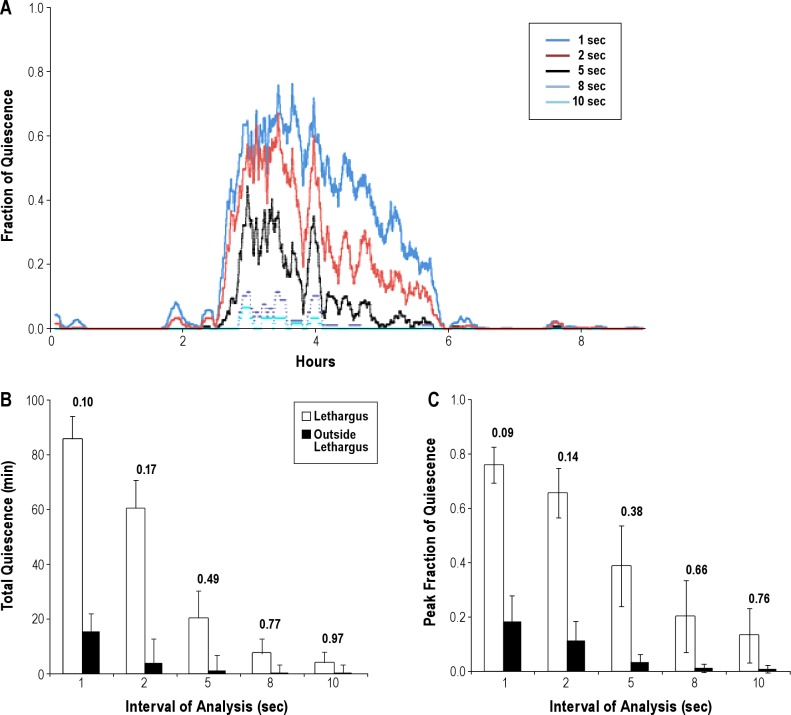
We tested the effect of various intervals of subtraction (τ) on quiescence during lethargus of the same animals. We measured the total quiescence, the duration of lethargus, and the peak fraction of quiescence in a 10-min period. The peak fraction of quiescence is a measure of the maximum degree of consolidation of quiescence and has been previously shown to be influenced by the history of prior wakefulness.2 A short interval of subtraction allows for detection of shorter quiescent periods, which enables the analysis of the microarchitecture of the lethargus period. However, when the interval of subtraction is excessively short, quiescence may be detected even outside lethargus, during brief pauses in locomotion. We acquired images of 19 wild-type worms at 1-sec intervals. We then performed the frame subtraction analysis with τ = 1, 2, 5, 8, and 10-sec intervals. Figure 3A depicts the fraction of quiescence for one worm as a function of time, with the analysis being carried out at different intervals of subtraction (see legend). As the interval of subtraction increases, the apparent fraction of quiescence reduces. Figures 3B and 3C depict, respectively, the total quiescence and peak fraction of quiescence as functions of the interval of subtraction (τ) during lethargus (hollow bars) and outside lethargus (black bars).
Figure 3.
The effect of interval of frame subtraction on quiescence. (A) Fraction of quiescence in a 10-min moving window of the data from the same worm analyzed at variable intervals of subtraction. (B) Mean total quiescence during lethargus and outside lethargus as a function of different subtraction intervals. Error bars denote standard deviation (SD). N = 19. Values above the bars are the coefficients of variation (CVs). (C) Mean peak fraction of quiescence during lethargus and outside lethargus as a function of different subtraction intervals. Error bars denote SD. N = 19. Values above the bars are the CVs.
At the 10-sec subtraction interval, which is the interval used in our prior analysis of worms on an agar surface2 as well as in the analysis of worms in artificial dirt,5 both the total quiescence as well as the peak fraction of quiescence were drastically reduced in the CiD method. Total quiescence and peak fraction of quiescence in L4 lethargus of worms cultured on an agar surface were, respectively, 46.6 ± 16.4 min and 0.60 ± 0.06 (mean ± SD). In contrast, these values of worms cultivated in droplets were 4.0 ± 3.9 min and 0.13 ± 0.10 (Figure 3). The peak fraction of quiescence obtained using a 1- or 2-sec interval of subtraction for analysis of quiescence of worms cultured in liquid droplets (Figure 3C) were more comparable to values obtained with a 10-sec interval of subtraction of worms cultured on agar. The total quiescence (Figure 3B) and the peak fraction of quiescence (Figure 3C) decreased exponentially as the subtraction time increased. The morphology of the fraction of quiescence trace, however, was independent of the interval of subtraction when this interval was < 5 seconds. Thus, we selected to use an interval of subtraction of 1 second.
In addition to increasing the ability to detect quiescence, we found that the coefficient of variation using the 1-second interval of subtraction was the lowest for both the peak quiescence and the total quiescence parameters (Figures 3B and 3C). Lower variance increases the power to detect effects of perturbations. Finally, using a short interval of subtraction permits the future assessment of the micro-architecture of lethargus. For these reasons, all future recordings were analyzed with a 1-sec interval of subtraction.
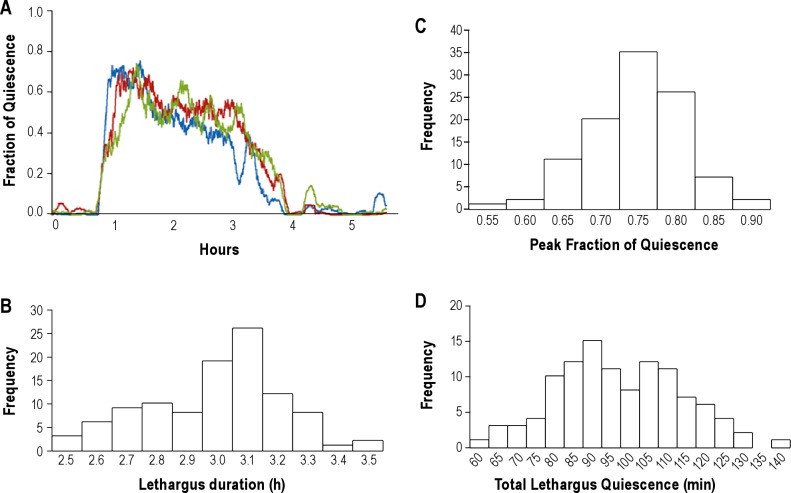
Using a 1-sec subtraction interval, we compared lethargus quiescence recordings of 104 wild-type animals. The quiescence patterns were highly reproducible among worms, as demonstrated by 3 representative worms in Figure 4A and the distributions of values (Figures 4B-D). Figures 4B, 4C, and 4D, depict, respectively, the probability distributions of the duration of lethargus quiescence, the peak fraction of quiescence, and the total quiescence. The 3 animals exhibited remarkably similar signatures of lethargus (Figure 4A), with the quiescence fraction peaking early during lethargus, and then gradually declining. Among these 104 animals, the average duration of lethargus quiescence was (mean ± SD) 3.04 ± 0.22 h (Figure 4B), the average peak fraction of quiescence was 0.77 ± 0.07 (Figure 4C), and the average total time spent quiescent was 97.92 ± 15.29 min (Figure 4D). These summary statistics show that duration of L4 lethargus quiescence in solution (3.04 ± 0.22 h) is 17% longer (P = 0.05, 2-tailed Student t-test) than the duration of L4 lethargus quiescence measured on an agar surface at 2.6 ± 0.54 (mean ± SD) hours.2 While there is a large reduction in total quiescence in liquid in comparison to agar, the duration of the stage is only slightly increased, suggesting that the main effect of the liquid environment is to reduce the quiescence associated with lethargus with little effect on the duration of the stage.
Figure 4.
Analysis of data from wild-type animals. (A) Fraction of quiescence in a 10-min moving window of 3 individual wild-type worms using a 1-sec interval of subtraction. The x-axis of the 3 traces was aligned so that the beginning of L4 lethargus was the same for the 3 traces. (B) Distribution of lethargus duration among 104 wild-type worms. The distribution did not deviate from normality (P > 0.05, Shapiro-Wilk Test, with Bonferroni post hoc correction).48 (C) Distribution of peak fraction of quiescence among 104 wild-type worms. The distribution did not deviate from normality (P > 0.05, Shapiro-Wilk Test, with Bonferroni post hoc correction). (D) Distribution of total quiescence in L4 lethargus among 104 wild-type worms. The distribution did not deviate from normality (P > 0.05, Shapiro-Wilk Test, with Bonferroni post hoc correction).
In summary, the CiD method for measuring lethargus quiescence provides high-quality, reproducible data, which shows comparable lethargus durations but reduced overall lethargus quiescence when compared to data obtained on agar.
Effect of Food Concentration on Behavior and Imaging in the Droplets
The availability of sufficient nutrition in the droplet was of concern given the importance of food intake on the growth and development of C. elegans. We used HB101 as a food source since it has been reported to be preferred by worms and to support better growth than the more popular OP50 bacteria strain.14,28 We tested the effect of bacterial concentration on quiescence measurements. Figure 5A shows photographs of droplets with various bacterial concentrations: 1X (Bacteria grown overnight to stationary phase), 4X, 15X, and 60X. At high bacterial concentration (60X), the transparency of the drop is impaired. Figures 5B and 5C depict, respectively, the duration of lethargus (hours) and the total quiescence time as functions of bacterial concentration. Figures 5B and 5C show that once the food supply exceeded 1x, the results were nearly independent of the food concentration.
Figure 5.
Effect of food concentration on lethargus quiescence. (A) The effect of food on optical transparency. All images were taken with the same light intensity and same camera exposure time. The concentration of the HB101 bacteria is shown above (1x and 4x) and below (15x and 60x) each of the 4 images. (B) The effect of food concentration on the mean and standard deviation (error bars) of the duration of lethargus. Value of each bar is shown inside the bar. N = 24 for 1X, 4X, and 60X concentrations and = 104 for 15X concentration. (C) The effect of food concentration on the mean and standard deviation (error bars) of the total lethargus quiescence. Value of each bar is shown inside the bar. N = 24 for 1X, 4X, and 60X concentrations and = 104 for 15X concentration. (D) In the absence of food, episodic quiescence outside of lethargus (arrows) is observed.
When we omitted bacteria from the solution entirely, we observed lengthy episodes of quiescence outside of lethargus (Figure 5D) as reported previously.29 Figure 5D should be contrasted with Figure 4A, which shows that in the presence of adequate food supply, there are no lengthy episodes of quiescence outside lethargus.
The CiD Method Can Be Used To Identify Lethargus Quiescence Mutants
To determine whether the CiD approach for measuring behavioral quiescence can be used to identify mutant behavior, we measured the quiescence of mutant animals. Figure 6A depicts the fraction of quiescence as a function of time during lethargus of each of the 3 mutants: egl-4(n479), kin-2(ce179), and acy-1(ce2). In each case, 3 representative traces of different animals were included to demonstrate the reproducibility of the data. Figures 6B and 6C depict, respectively, the total lethargus quiescence and the peak fraction of quiescence of each strain.
Figure 6.
Analysis of mutants using CiD. (A) Fraction of quiescence in a 10-min moving window of 3 individual worms of each of 3 genotypes using a 1-sec interval of subtraction. The x-axis of each series of 3 traces was aligned so that the beginning of L4 lethargus was the same for all worms. Data for wild-type animals is the same data shown in Figure 4A. (B) Total lethargus quiescence. Each point denotes a value from a single worm. The horizontal bars denote means of the distributions. *denotes significantly different from wild-type at P < 0.001 (2-tailed Wilcoxon rank sum test). N(wild type) = 104, N(egl-4) = 18, N(kin-2) = 24, N(acy-1) = 19. Mean ± SD (wild-type) = 97.9 ± 15.3, mean ± SD (egl-4) = 110.2 ± 25.5, mean ± SD (acy-1) = 36.6 ± 25.5, mean ± SD (kin-2) = 32.9 ± 25.2. (C) Peak fraction of quiescence. Each point denotes a value from a single worm. The horizontal bars denote means of the distribution. There was no significant difference between the mutants and wild-type worms (P > 0.05, 2-tailed Wilcoxon rank sum test). Number of animals is shown in B. Mean ± SD (wild-type) = 0.77 ± 0.07, mean ± SD (egl-4) = 0.83 ± 0.11, mean ± SD (acy-1) = 0.73 ± 0.13, mean ± SD (kin-2) = 0.87 ± 0.16.
The first mutant we assessed, egl-4(n479), was previously shown to have reduced quiescence during L4 lethargus both on an agar surface2 as well as on a PDMS chip configured with artificial dirt.5 Furthermore, egl-4 loss-of-function mutants are hyper responsive during lethargus, suggesting more wake-like behavior.2 Finally, egl-4 has been shown to be necessary for behavioral quiescence induced by manipulating the satiety state of the animal,2 by over-expressing epidermal growth factor,4 or by over-expressing the Notch co-ligand OSM-11.5 In contrast to these prior results, when measured with the CiD method, quiescence parameters of egl-4(n479) loss-of-function mutants were not significantly different from those of wild-type animals (Figure 4). An additional egl-4 loss-of-function allele, ks62, also showed no difference (P = 0.7, Wilcoxon rank sum test) in total quiescence (108.4 ± 15.6 min, N = 9) from wild-type controls (104.2 ± 11.7 min, N = 9) that were measured simultaneously in CiD. Therefore, it appears that ambient conditions exert a significant effect on behavioral quiescence.
Other mutants of interest are those in which signaling by cAMP-dependent protein kinase (PKA) is predicted to be increased. PKA is known to be a major cellular target of cAMP, and both PKA and the PKA substrate CREB have been implicated as wake-promoting in Drosophila30,31 as well as in mice.32 Gain-of-function mutants in the gene acy-1, which encodes an adenylate cyclase, have been reported to be hyper responsive in the adult stage15,33 as well as in the L4 lethargus stage.2 Loss-of-function mutants in the gene kin-2, which encodes the regulatory subunit of PKA,34 have been reported to be hyperactive and hyperresponsive as adults.15,33 Our prior attempts to measure L4 lethargus quiescence of these PKA signaling mutants on an agar surface have been unsuccessful, due to these hyperactive mutants escaping from the field of view of the camera.2 Therefore, the CiD method, in which worms are confined in the droplet, provides an opportunity to measure behavioral quiescence in these hyperactive mutants.
We measured quiescence of worms harboring an acy-1 gain-of-function mutation and of worms harboring a kin-2 loss-of-function mutation. Both mutant strains showed marked reduction of total quiescence (Figure 6B). Interestingly, the peak fraction of quiescence was not significantly reduced in these mutants (Figure 6C), indicating that the 2 parameters, peak fraction of quiescence and total quiescence, are controlled by partially distinct genetic mechanisms. The reduced quiescence of kin-2 mutants (39.6 ± 17.9 min) was rescued to values greater than that observed in wild-type animals by transgenic expression of kin-2 under the control of the nervous system-specific promoter Punc-119 (268 ± 60.2 min, Figure S3), supporting the notion that it is the kin-2 mutation and not a background mutation that accounts for the reduced quiescence phenotype in that strain. Further, this transgenic experiment demonstrates that kin-2 likely functions in the nervous system to regulate behavioral quiescence. In addition to its effect on behavioral quiescence, the Punc-119:kin-2 transgene also rescued the defect in sensory arousal threshold during lethargus observed in kin-2(ce179) mutants (Table S2). In contrast to its effects in the nervous system, expression of kin-2 in the hypodermis did not rescue the enhanced sensory responsiveness during lethargus of kin-2 mutants (Table S2).
In addition to assessing effects of genetic manipulations predicted to reduce lethargus quiescence, we also assessed the effects of a genetic manipulation predicted to increase quiescence during the adult stage, when animals are typically active. Van Buskirk and Sternberg reported that animals induced to express elevated levels of epidermal growth factor, which is encoded by the gene lin-3, show reduced movement on an agar surface.4 We asked whether this effect is preserved in CiD. Indeed, within 2 hours after heat shock, the stimulus used to induce lin-3 over-expression, adult animals show greatly increased quiescence in comparison to control animals of the same genotype that were not heat-shocked as well as compared to heat-shocked wild-type animals (Figure S4).
Based on these analyses, we conclude that the CiD method is capable of detecting abnormal quiescence behavior, both reduced quiescence during lethargus (in the case of kin-2 and acy-1 mutants) and increased quiescence outside lethargus (in the case of kin-2 transgenic animals and lin-3 over-expressing animals), and that certain mutants behave differently on an agar surface than in liquid.
DISCUSSION
We here described the use of a droplet-based reactor to study C. elegans behavior for prolonged periods of time. The Caenorhabditis-in-Drop (CiD) method offers advantages over prior methods used to record behavior over the course of several hours. First, since the animal is confined to a drop-based reactor, it cannot escape from the field of view of the camera. This is an advantage in the study of hyperactive mutants, which often leave the field of view of the camera when housed on an agar surface (our unpublished observations). Using this method, we were successful in measuring, for the first time, quiescence in kin-2(ce179) and in acy-1(ce2) mutants, which are hyper-active. These new data firmly establish the cAMP-dependent protein kinase A (PKA) signaling pathway as wake-promoting in C. elegans. Given the compelling data showing that in both Drosophila30,35 and mammals32,36 genes in this pathway are wake-promoting, our findings support the idea that lethargus quiescence behavior is sleep-like.
Animals with loss-of-function mutations for the gene egl-4, which has been shown by several groups independently to be required for proper behavioral quiescence,2,4,5,37showed no reduction of quiescence using CiD method. One possible explanation for these discrepant results is that egl-4 mutants, which are hyperresponsive to olfactory and gustatory stimuli due to failure in chemosensory adaptation,38,39 are susceptible to ambient perturbations when housed on an agar surface but are shielded from such chemical perturbations when housed in droplets. This observation highlights the effects of the environment on lethargus quiescence and indicates that effects of genotype on behavioral quiescence phenotypes are sensitive to the environmental context. This sensitivity to the environment demonstrates another similarity between lethargus quiescence and sleep behavior in other animals, which are known to engage in sleep and sleep-like states in particular environmental locations and with particular postures.18,40,41 Regardless of the explanation, this should raise a note of caution in the interpretation of both positive and negative results. That is, analysis of genes and circuits regulating behavior on an agar surface may be different from those regulating behavior in droplets.
We observed reduced variance in the quiescence in droplets in comparison to an agar surface. This increased reliability of the behavior may be partially explained by a more consistent environment in the droplet than on the agar surface. Reduced variance is an advantage when pursuing mutants for a quantitative trait such as behavioral quiescence.
Finally, the CiD offers higher throughput for automated analysis of quiescent behavior than previously reported methods. Only a single worm was monitored on agar,2 and prior microfluidic designs included either five L4 worms in the field of view5 or a single L1 worm in the field of view.42 Here, we report the routine monitoring of 24 worms with a single camera. In this study, our use of a relatively low-resolution camera limited the area of image acquisition and the number of drops that could be imaged concurrently while preserving spatial resolution. Larger areas and a greater number of drops can be imaged with the use of a higher resolution camera. With such a future modification, one should be able to simultaneously image hundreds of worms in parallel—a number that is sufficiently large to begin systematic assessment of genetic perturbations on leth-argus quiescence. Future improvements in throughput of this method can be achieved by automating the encapsulation of the worms in droplets,19,43 and by incorporating a motorized camera or stage to allow the imaging of more than one field of view. The future recovery of animals with mutant behavior can be achieved by developing means to tag and recover single animals from the chip. Such future improvements hold potential for massive expansion of analysis throughput to that of thousands of worms.
Two caveats to this method are important to note. First, we observed a reduced longevity in the CiD device in comparison to longevity on an agar surface. The longevity in CiD may limit certain applications, for example, the analysis of circadian rhythms. Based on our observation of increased survival in a ten-fold greater volume, we excluded the possibilities that the liquid habitat or the exposure to mineral oil cause reduced longevity. We suggest three possible explanations for the reduced long-term survival in CiD. First, it is possible, albeit unlikely, that the demise of the worms after 4-5 days is caused by depletion of nutrients in the droplet. While our gas exchange model suggests that oxygen supply should not be limiting, the bacteria supplied in the droplet is finite and should eventually be depleted. Second, it is possible that toxic waste is accumulating in the droplet. Such waste is more likely to be aqueous than gaseous (see Supplementary). C. elegans is known to secrete pheromones and steroids, which can have complex effects on behavior and viability.44 In a small volume, such chemicals may accumulate and adversely affect survival. Finally, it is possible that confinement in a small volume is harmful to the animal. Prior observations have shown an adverse effect of solitary rearing of C. elegans.45 In the tiny volume of the CiD device, such solitary effects may be magnified. The identification of the mechanism that accounts for the reduced longevity will require additional experimentations. For certain multi-day future applications of the device, such as circadian rhythm experiments, refinements in the method will be required. Future modifications of the method may include the development of means to fuse droplets and thus modify the droplet contents during experiments.
Second, it is important to emphasize that we are measuring quiescence, or absence of movement, and not directly sleep. In addition to inactivity, sleep is defined by an elevated sensory arousal threshold and homeostatic response to deprivation of quiescence.46 There is already a precedent in the nascent C. elegans sleep literature of mutants that show increased quiescence yet reduced arousal threshold.5 Future experiments can make use of photo avoidant response47 to assess arousal threshold in the CiD device.
In summary, we describe a new method for long-term monitoring of C. elegans behavior. We here described the analysis only of behavioral quiescence associated in L4 lethargus. However, the same device can be used for monitoring L3 lethargus as well as for other long-term behaviors including swimming and egg-laying. The simplicity of device fabrication and of performing the experiment holds promise for easy adaptability of the method by other researchers.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMEMTS
The authors thank Mr. Robert Driver for performing sensory arousal experiments. Work for this study was performed at the University of Pennsylvania, Philadelphia, PA. This research was supported in part by UPENN ITMAT (UL1RR024134 from the National Center for Research Resources). Mr. Belfer was supported in part by the Pincus-Magaziner Family Undergraduate Research and Travel Grant at the University of Pennsylvania, Dr. Chuang was supported by 100-2218-E-006-036 from the Taiwan National Science Council, and Dr. Raizen was supported by R01 NS064030 from the NIH and by a NARSAD Young Investigator Award. Strains used in this study were obtained from Dr. Cheryl Van Buskirk and from the Caenorhabditis Genetics Center (CGC), which is funded by the National Institute of Health - Office of Research Infrastructure Programs (P40 OD010440).
NOTE ADDED IN PROOF
Iwanir et al. recently reported a reduced quiescence phenotype of acy-2(ce2) mutants (Iwanir S, Tramm N, Nagy S, Wright C, Ish D, Biron D. The microarchitecture of C. elegans behavior during lethargus: homeostatic bout dynamics, a typical body posture, and regulation by a central neuron. SLEEP 2013;36:385-395.).
SUPPLEMENTAL MATERIAL
Caenorhabditis-in-Drop for Monitoring C. elegans Quiescent Behavior
Samuel J. Belfer, Han-Sheng Chuang, Benjamin L. Freedman, Jinzhou Yuan, Michael Horton, Haim H. Bau, and David M. Raizen
Estimation of Gas Exchange in a Drop
Baltz et al's analytical model1 is used here to estimate the oxygen (O2) supply into an aqueous drop. The model assumes that the drop has a hemispherical shape of radius r1 and rests on an impermeable surface. The drop is enveloped with a concentric hemispherical shell of mineral oil of radius r2 as shown in Figure S1. The worm is approximated as a concentric hemisphere of radius a. The model allows for spherical symmetry. The gas concentration (C) distribution of species j is governed by the one-dimensional diffusion equation:
 |
where r is the radial coordinate and Di is the diffusion coefficient in medium i. We use the subscripts “w” and “o” to denote, respectively, the water drop and the surrounding oil shell. The saturation concentrations of the gas in the water and oil are denoted, respectively, as Sw and So.
A model to estimate gas exchange between a water drop and the surrounding air
Oxygen Supply
When we solve for the oxygen concentration distribution, we specify the oxygen flux at the worm's surface
 |
In the above, U is the worm's oxygen consumption. At the liquid – oil interface, we assume local equilibrium
where K is the partition coefficient. In what follows, we will approximate the partition coefficient as . Additionally, mass conservation requires flux continuity
 |
At the outer surface of the oil shell, the oxygen concentration in the oil is at equilibrium with the ambient air.
We assume that initially, both the water and oil are fully saturated with oxygen
 |
The steady state solution of equations (S1)-(S5) can be readily obtained in closed form.
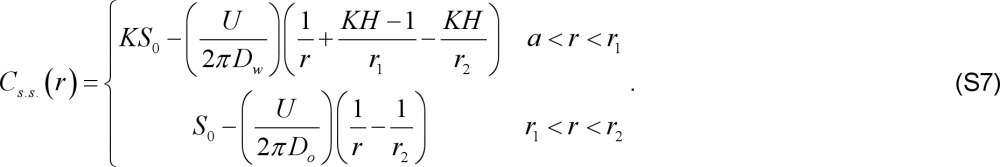 |
In the above, H = Dw /Do is the ratio of the diffusion coefficients. For later use, we also write the expression for the steady state oxygen concentration in the oil at the water-oil interface:
 |
where . One condition for a steady-state solution to exist is . When χ<<1, one can assume that in the oil region (r>r1) C(r,t) ∼ S0.
We use the properties of oxygen documented in Table S1 and the maximum oxygen consumption rate of a worm is 10.5×10-12 mol/min as reported by Suda et al.2 In our case, χ = 0.006. The steady-state concentration distribution of oxygen in the water-oil system is depicted in Figure S2a when r1 = 0.5mm and r2 = 1.5mm and one can assume that the oxygen concentration in the oil is nearly uniform at the saturation value. In other words, the oil does not provide much resistance to the oxygen transport. The water drop can be viewed as if it were nearly in direct contact with air and interfacial conditions (S3) and (S4) can be replaced with C(r1-,t)~Sw.
Properties of oxygen and carbon dioxide.
The steady state oxygen (A) and CO2 (B) concentrations as functions of position when the worm consumes maximum amount of oxygen (solid line) and in the absence of a worm (circles). The insets in (A) are magnified versions of the sub-sections of the curve.
Given the foregoing, the time constant of the system is approximately r12/Dw~104s or three hours. This estimate of the time constant was verified with direct numerical simulations carried out with the multi-physics, finite element program COMSOLTM. The numerical simulations accounted fully for the oxygen transport in the oil shell and demonstrated that a nearly steady state solution was reached within three hours. This gives further support to the hypothesis that there is a sufficient oxygen supply to sustain the worm. If the oxygen concentration in the water were to drop below the lethal limit, the worms should be dead within a few hours. This was not the case in our experiments. Hence we conclude that the worms in the drops had adequate oxygen supply.
Carbon Dioxide Removal
Another concern is the possibility of accumulating waste products in the water drop due to the nematode's metabolic activity. One such waste product is CO2. We used a model similar to the one described in the previous section to estimate the concentration distribution of carbon dioxide (CO2) in the system. We assume that the carbon dioxide at the oil surface Co(r2) = 1.28 mM is equal to the concentration of CO2 in air. This value is well below the solubility limit of CO2 in oil (Table S1). We also assume that the rate of CO2 production (on molar basis) is equal to the oxygen consumption. The CO2 concentrations in the water and oil at the water-oil interface are related through the partition coefficient K (equation S3).
The steady-state concentration distribution of CO2 as a function of radial position is depicted in Figure 2Sb. The maximum estimated concentration in the water droplet is 0.88 mM. This concentration is obtained within about 3 hours after the insertion of the worm in the drop. If this concentration were lethal to the worm, the worm should have perished within a few hours. This is, however, not the case in our experiments. Hence we conclude that CO2 is removed at sufficient rate to sustain the worm.
Compared with the reported concentration (> 9% CO2 in air, ~3.68 mM) that may disrupt a worm's physiological development3, the metabolic gas waste doesn't seem to be significant enough to disturb the worm's life.
An example of the fraction of quiescence of one kin-2(ce179); Punc-119:kin-2 worm. Recording was begun in the mid L4 stage and continued for 10 hours. Because the start and end of lethargus are not clearly defined in worms of this genotype, the total quiescence in the 10-hour recording (268 ± 60.2 minutes) was measured and compared to the total quiescence in a 10-hour recording of equivalent age kin-2 mutants (39.6 ± 17.9 minutes). Compare graph to that of kin-2 in Figure 6A.
Sensory response during lethargus of kin-2(ce179) and transgenic animals.
Animals were identified in L4 lethargus based on cessation of locomotion and feeding on an NGM plates fully covered with a lawn of DA837 bacteria. They were stimulated with blue light using a Leica MZ16 stereomicroscope set at 8x objective magnification and equipped with a GFP filter (excitation wavelength: 450-490 nm) in the microscope head. Dim white light was used to continuously illuminate the worms via the microscope base and intermittent epi-illumination with blue light was controlled with a mechanical shutter on an EL600 metal halide light source. At the magnification and filter settings used, the irradiance was 0.3 mW/mm2. A photoavoidant response6 was defined as a movement of the animal for one half of its body length. Experiments were performed on the same day by the same investigator who was blinded to genotype.
Increased quiescence in response to induction of LIN-3C over-expression using a heat stimulus. Shown are representative examples of behavioral quiescence analysis of adult wild-type worms or adult worms carrying the extrachromosomal array hs:lin-3C who were either maintained at 20 degrees (left graphs) or were heat-shocked in a 33 degree water bath for 20 minutes immediately prior to loading (right graph). The loading process into the CiD device was completed in 20 minutes. The 3 colors represent 3 individual worms. The mean ± standard deviation of the total quiescence in 10 hours of 10 non-heat-shocked N2 wild-type animals was 32 ± 72; of 10 heat-shocked N2 wild-type animals was 13 ± 7 minutes (P = 0.18, Wilcoxon Rank Sum Test); of 12 non-heat-shocked LIN-3C animals was 11 ± 20; and of 12 heat-shocked LIN-3C animals was 347 ± 81 minutes (P < 0.00001, Wilcoxon Rank Sum Test).
Supplementary References
- 1.Baltz JM, Biggers JD. Oxygen transport to embryos in microdrop cultures. Mol Reprod Dev. 1991;28:351–5. doi: 10.1002/mrd.1080280407. [DOI] [PubMed] [Google Scholar]
- 2.Suda H, Shouyama T, Yasuda K, Ishii N. Direct measurement of oxygen consumption rate on the nematode Caenorhabditis elegans by using an optical technique. Biochem Biophys Res Commun. 2005;330:839–43. doi: 10.1016/j.bbrc.2005.03.050. [DOI] [PubMed] [Google Scholar]
- 3.Sharabi K, Hurwitz A, Simon AJ, et al. Elevated CO2 levels affect development, motility, and fertility and extend life span in Caenorhabditis elegans. PNAS. 2009;106:4024–9. doi: 10.1073/pnas.0900309106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Emsley J. Oxygen. Nature's Building Blocks: An A-Z Guide to the Elements. Oxford, England, UK: Oxford University Press; 2001. pp. 297–304. [Google Scholar]
- 5.Stokes YM. Quantifying oxygen diffusion in paraffin oil used in oocyte and embryo culture. Mol Reprod Dev. 2009;76:1178–87. doi: 10.1002/mrd.21089. [DOI] [PubMed] [Google Scholar]
- 6.Edwards SL, Charlie NK, Milfort MC, et al. A novel molecular solution for ultraviolet light detection in Caenorhabditis elegans. PLoS Biol. 2008;6:e198. doi: 10.1371/journal.pbio.0060198. [DOI] [PMC free article] [PubMed] [Google Scholar]
REFERENCES
- 1.Singh RN, Sulston JE. Some observations on moulting in Caenorhabditis elegans. Nematologica. 1978;24:63–71. [Google Scholar]
- 2.Raizen DM, Zimmerman JE, Maycock MH, et al. Lethargus is a Caenorhabditis elegans sleep-like state. Nature. 2008;451:569–72. doi: 10.1038/nature06535. [DOI] [PubMed] [Google Scholar]
- 3.Monsalve GC, Van Buskirk C, Frand AR. LIN-42/PERIOD controls cyclical and developmental progression of C. elegans molts. Curr Biol. 2012;21:2033–45. doi: 10.1016/j.cub.2011.10.054. [DOI] [PubMed] [Google Scholar]
- 4.Van Buskirk C, Sternberg PW. Epidermal growth factor signaling induces behavioral quiescence in Caenorhabditis elegans. Nat Neurosci. 2007;10:1300–7. doi: 10.1038/nn1981. [DOI] [PubMed] [Google Scholar]
- 5.Singh K, Chao MY, Somers GA, et al. C. elegans Notch signaling regulates adult chemosensory response and larval molting quiescence. Curr Biol. 2011;21:825–34. doi: 10.1016/j.cub.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lockery SR, Lawton KJ, Doll JC, et al. Artificial dirt: microfluidic substrates for nematode neurobiology and behavior. J Neurophysiol. 2008;99:3136–43. doi: 10.1152/jn.91327.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bringmann H. Agarose hydrogel microcompartments for imaging sleep-and wake-like behavior and nervous system development in Caenorhabditis elegans larvae. J Neurosci Methods. 2011;201:78–88. doi: 10.1016/j.jneumeth.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 8.Pipper J, Zhang Y, Neuzil P, Hsieh TM. Clockwork PCR including sample preparation. Angew Chem Int Ed Engl. 2008;47:3900–4. doi: 10.1002/anie.200705016. [DOI] [PubMed] [Google Scholar]
- 9.Clausell-Tormos J, Lieber D, Baret JC, et al. Droplet-based microfluidic platforms for the encapsulation and screening of mammalian cells and multicellular organisms. Chem Biol. 2008;15:427–37. doi: 10.1016/j.chembiol.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 10.Srinivasan V, Pamula VK, Fair RB. An integrated digital microfluidic lab-on-a-chip for clinical diagnostics on human physiological fluids. Lab Chip. 2004;4:310–5. doi: 10.1039/b403341h. [DOI] [PubMed] [Google Scholar]
- 11.Shi W, Wen H, Lu Y, Shi Y, Lin B, Qin J. Droplet microfluidics for characterizing the neurotoxin-induced responses in individual Caenorhabditis elegans. Lab Chip. 2010;10:2855–63. doi: 10.1039/c0lc00256a. [DOI] [PubMed] [Google Scholar]
- 12.Boyer HW, Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969;41:459–72. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- 13.Merek EL. Estimating the size and concentration of unicellular microorganisms by light scattering. Appl Microbiol. 1969;17:219–21. doi: 10.1128/am.17.2.219-221.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schade MA, Reynolds NK, Dollins CM, Miller KG. Mutations that rescue the paralysis of Caenorhabditis elegans ric-8 (synembryn) mutants activate the G alpha(s) pathway and define a third major branch of the synaptic signaling network. Genetics. 2005;169:631–49. doi: 10.1534/genetics.104.032334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trent C, Tsuing N, Horvitz HR. Egg-laying defective mutants of the nematode Caenorhabditis elegans. Genetics. 1983;104:619–47. doi: 10.1093/genetics/104.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirose T, Nakano Y, Nagamatsu Y, Misumi T, Ohta H, Ohshima Y. Cyclic GMP-dependent protein kinase EGL-4 controls body size and lifespan in C elegans. Development. 2003;130:1089–99. doi: 10.1242/dev.00330. [DOI] [PubMed] [Google Scholar]
- 18.Zimmerman JE, Raizen DM, Maycock MH, Maislin G, Pack AI. A video method to study Drosophila sleep. Sleep. 2008;31:1587–98. doi: 10.1093/sleep/31.11.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brouzes E, Medkova M, Savenelli N, et al. Droplet microfluidic technology for single-cell high-throughput screening. Proc Natl Acad Sci U S A. 2009;106:14195–200. doi: 10.1073/pnas.0903542106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mattiasson B, Adlercreutz P. Per fluorochemicals in biotechnology. Trends Biotechnol. 1987;5:250–4. [Google Scholar]
- 21.Sharabi K, Hurwitz A, Simon AJ, et al. Elevated CO2 levels affect development, motility, and fertility and extend life span in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2009;106:4024–9. doi: 10.1073/pnas.0900309106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khanna N, Cressman CP, 3rd, Tatara CP, Williams PL. Tolerance of the nematode Caenorhabditis elegans to pH, salinity, and hardness in aquatic media. Arch Environ Contam Toxicol. 1997;32:110–4. doi: 10.1007/s002449900162. [DOI] [PubMed] [Google Scholar]
- 23.Hulme SE, Shevkoplyas SS, McGuigan AP, Apfeld J, Fontana W, Whitesides GM. Lifespan-on-a-chip: microfluidic chambers for performing lifelong observation of C. elegans. Lab Chip. 2010;10:589–97. doi: 10.1039/b919265d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hosono R. Sterilization and growth inhibition of Caenorhabditis elegans by 5-fluorodeoxyuridine. Exp Gerontol. 1978;13:369–74. doi: 10.1016/0531-5565(78)90047-5. [DOI] [PubMed] [Google Scholar]
- 25.Gray J, Lissmann HW. The locomotion of nematodes. J Exp Biol. 1964;41:135–54. doi: 10.1242/jeb.41.1.135. [DOI] [PubMed] [Google Scholar]
- 26.Korta J, Clark DA, Gabel CV, Mahadevan L, Samuel AD. Mechanosensation and mechanical load modulate the locomotory gait of swimming C. elegans. J Exp Biol. 2007;210(Pt 13):2383–9. doi: 10.1242/jeb.004572. [DOI] [PubMed] [Google Scholar]
- 27.Pierce-Shimomura JT, Chen BL, Mun JJ, Ho R, Sarkis R, McIntire SL. Genetic analysis of crawling and swimming locomotory patterns in C. elegans. Proc Natl Acad Sci U S A. 2008;105:20982–7. doi: 10.1073/pnas.0810359105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shtonda BB, Avery L. Dietary choice behavior in Caenorhabditis elegans. J Exp Biol. 2006;209:89–102. doi: 10.1242/jeb.01955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ghosh R, Emmons SW. Episodic swimming behavior in the nematode C. elegans. J Exp Biol. 2008;211(Pt 23):3703–11. doi: 10.1242/jeb.023606. [DOI] [PubMed] [Google Scholar]
- 30.Hendricks JC, Williams JA, Panckeri K, et al. A non-circadian role for cAMP signaling and CREB activity in Drosophila rest homeostasis. Nat Neurosci. 2001;4:1108–15. doi: 10.1038/nn743. [DOI] [PubMed] [Google Scholar]
- 31.Joiner WJ, Crocker A, White BH, Sehgal A. Sleep in Drosophila is regulated by adult mushroom bodies. Nature. 2006;441:757–60. doi: 10.1038/nature04811. [DOI] [PubMed] [Google Scholar]
- 32.Graves LA, Hellman K, Veasey S, Blendy JA, Pack AI, Abel T. Genetic evidence for a role of CREB in sustained cortical arousal. J Neurophysiol. 2003;90:1152–9. doi: 10.1152/jn.00882.2002. [DOI] [PubMed] [Google Scholar]
- 33.Harris G, Mills H, Wragg R, et al. The monoaminergic modulation of sensory-mediated aversive responses in Caenorhabditis elegans requires glutamatergic/peptidergic cotransmission. J Neurosci. 2010;30:7889–99. doi: 10.1523/JNEUROSCI.0497-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lu XY, Gross RE, Bagchi S, Rubin CS. Cloning, structure, and expression of the gene for a novel regulatory subunit of cAMP-dependent protein kinase in Caenorhabditis elegans. J Biol Chem. 1990;265:3293–303. [PubMed] [Google Scholar]
- 35.Joiner WJ, Crocker A, White BH, Sehgal A. Sleep in Drosophila is regulated by adult mushroom bodies. Nature. 2006;441:757–60. doi: 10.1038/nature04811. [DOI] [PubMed] [Google Scholar]
- 36.Vecsey CG, Baillie GS, Jaganath D, et al. Sleep deprivation impairs cAMP signalling in the hippocampus. Nature. 2009;461:1122–5. doi: 10.1038/nature08488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.You YJ, Kim J, Raizen DM, Avery L. Insulin, cGMP, and TGF-beta signals regulate food intake and quiescence in C. elegans: a model for satiety. Cell Metab. 2008;7:249–57. doi: 10.1016/j.cmet.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.L'Etoile ND, Coburn CM, Eastham J, Kistler A, Gallegos G, Bargmann CI. The cyclic GMP-dependent protein kinase EGL-4 regulates olfactory adaptation in C. elegans. Neuron. 2002;36:1079–89. doi: 10.1016/s0896-6273(02)01066-8. [DOI] [PubMed] [Google Scholar]
- 39.Jansen G, Weinkove D, Plasterk RH. The G-protein gamma subunit gpc-1 of the nematode C.elegans is involved in taste adaptation. Embo J. 2002;21:986–94. doi: 10.1093/emboj/21.5.986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tobler I. Phylogeny of sleep regulation. In: Kryger, Roth, Dement, editors. Principles and practice of sleep medicine. 3rd ed. Philadelphia PA: Saunders; 2001. pp. 72–81. [Google Scholar]
- 41.Hendricks JC, Finn SM, Panckeri KA, et al. Rest in Drosophila is a sleep-like state. Neuron. 2000;25:129–38. doi: 10.1016/s0896-6273(00)80877-6. [DOI] [PubMed] [Google Scholar]
- 42.Bringmann H. Agarose hydrogel microcompartments for imaging sleep-and wake-like behavior and nervous system development in Caenorhabditis elegans larvae. J Neurosci Methods. 2011;201:78–88. doi: 10.1016/j.jneumeth.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 43.Vijayakumar K, Gulati S, deMello AJ, Edel JB. Rapid cell extraction in aqueous two-phase microdroplet systems. Chem Sci. 2010;1:447–52. [Google Scholar]
- 44.Lee SS, Schroeder FC. Steroids as central regulators of organismal development and lifespan. PLoS Biol. 2012;10:e1001307. doi: 10.1371/journal.pbio.1001307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rose JK, Sangha S, Rai S, Norman KR, Rankin CH. Decreased sensory stimulation reduces behavioral responding, retards development, and alters neuronal connectivity in Caenorhabditis elegans. J Neurosci. 2005;25:7159–68. doi: 10.1523/JNEUROSCI.1833-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hendricks JC, Sehgal A, Pack AI. The need for a simple animal model to understand sleep. Prog Neurobiol. 2000;61:339–51. doi: 10.1016/s0301-0082(99)00048-9. [DOI] [PubMed] [Google Scholar]
- 47.Edwards SL, Charlie NK, Milfort MC, et al. A novel molecular solution for ultraviolet light detection in Caenorhabditis elegans. PLoS Biol. 2008;6:e198. doi: 10.1371/journal.pbio.0060198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shapiro SS, Wilk MB. An analysis of variance test for normality (complete samples) Biometrika. 1965;52:591–611. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A model to estimate gas exchange between a water drop and the surrounding air
Properties of oxygen and carbon dioxide.
The steady state oxygen (A) and CO2 (B) concentrations as functions of position when the worm consumes maximum amount of oxygen (solid line) and in the absence of a worm (circles). The insets in (A) are magnified versions of the sub-sections of the curve.
An example of the fraction of quiescence of one kin-2(ce179); Punc-119:kin-2 worm. Recording was begun in the mid L4 stage and continued for 10 hours. Because the start and end of lethargus are not clearly defined in worms of this genotype, the total quiescence in the 10-hour recording (268 ± 60.2 minutes) was measured and compared to the total quiescence in a 10-hour recording of equivalent age kin-2 mutants (39.6 ± 17.9 minutes). Compare graph to that of kin-2 in Figure 6A.
Sensory response during lethargus of kin-2(ce179) and transgenic animals.
Increased quiescence in response to induction of LIN-3C over-expression using a heat stimulus. Shown are representative examples of behavioral quiescence analysis of adult wild-type worms or adult worms carrying the extrachromosomal array hs:lin-3C who were either maintained at 20 degrees (left graphs) or were heat-shocked in a 33 degree water bath for 20 minutes immediately prior to loading (right graph). The loading process into the CiD device was completed in 20 minutes. The 3 colors represent 3 individual worms. The mean ± standard deviation of the total quiescence in 10 hours of 10 non-heat-shocked N2 wild-type animals was 32 ± 72; of 10 heat-shocked N2 wild-type animals was 13 ± 7 minutes (P = 0.18, Wilcoxon Rank Sum Test); of 12 non-heat-shocked LIN-3C animals was 11 ± 20; and of 12 heat-shocked LIN-3C animals was 347 ± 81 minutes (P < 0.00001, Wilcoxon Rank Sum Test).