Abstract
Ion and water transport by the kidney is continually adjusted in response to physiological cues. Selective endocytosis and endosomal trafficking of ion transporters are increasingly appreciated as mechanisms to acutely modulate renal function. Here, we discuss emerging paradigms in this new area of investigation.
Endosomes have emerged as a key intracellular sorting site in kidney cells. Internalized transmembrane proteins that enter these compartments can be targeted for recycling to the cell surface, for transport to the lysosome, for transcytosis, or for retrograde transport to the trans-Golgi network (TGN). The default trafficking pathway that is followed for a given protein depends on its inherent targeting signals and the availability and localization of adaptors that recognize this information. In recent years, it has become evident that the postendocytic fate of surface receptors and ion transporters is highly regulated within this pathway. A variety of posttranslational modifications can alter or create new endocytic signals to acutely enable proteins to engage different trafficking routes. In this review, we describe endocytic pathways and the machinery that regulate these routes, with emphasis on emerging paradigms that highlight the complexity of these pathways and their participation in the modulation of renal ion transport. We have used examples from differentiated kidney cells where possible but note that many of the studies we discuss were performed in nonpolarized cells, where endocytic traffic is less complex than in polarized cells.
Endocytosis and Postendocytic Pathways
Cell surface receptors, transporters, and channels can be internalized by several different mechanisms (see Ref. 13 for review), of which the clathrin-dependent pathway is the best understood. In each case, endocytosis involves the invagination and fission of the plasma membrane to generate cytoplasmic vesicles that ultimately fuse with early sorting endosomes (EEs; FIGURE 1A). The slightly acidic lumenal pH of EEs favors dissociation of internalized ligands from membrane receptors. In EEs, proteins are sorted for recycling, degradation, retrograde transport to the TGN, or transcytosis to the opposing cell surface domain. Lumenal contents of EEs, including dissociated ligands, are sorted from recycling lipid and membrane proteins at this stage: membrane receptors and other proteins enter tubular extensions that can return to the surface rapidly and directly (fast recycling) or after convergence in juxtanuclear recycling endosomes. Motor proteins including myosins play a role in protein delivery from recycling endosomes to the cell surface. Cargoes destined for degradation (e.g., lumenal contents or membrane proteins that are targeted for degradation by ubiquitination) are retained in sorting endosomes. EEs mature to become late endosomes/multivesicular bodies (LEs/MVBs) by invagination of the limiting membrane to form lumenal vesicles. MVBs transiently fuse with lysosomes to deliver their intralumenal contents and then re-form.
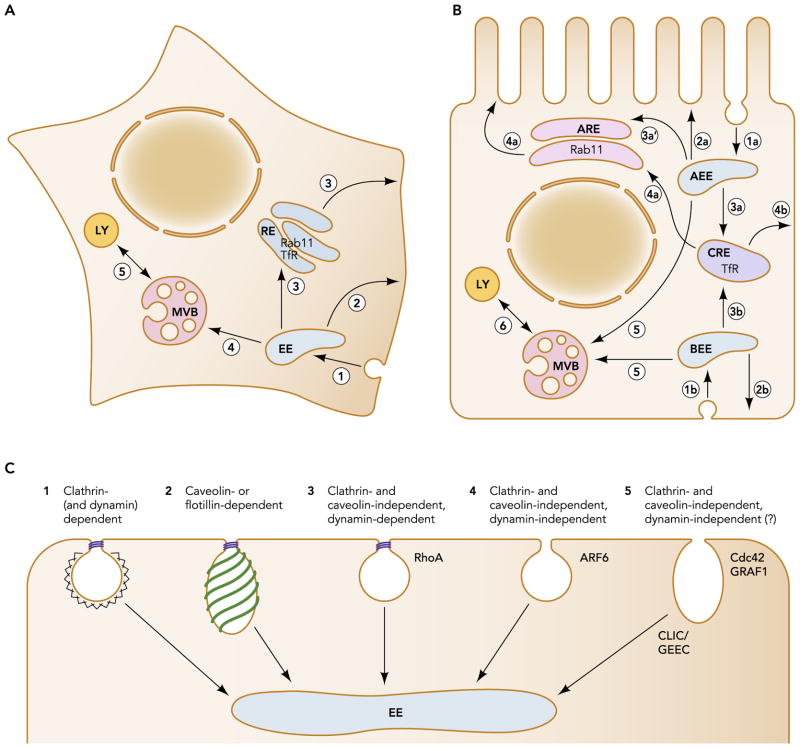
FIGURE 1. The endocytic pathway and entry portals in undifferentiated and polarized cells.
A: in nonpolarized cells, cargo internalized through clathrin coated pits (1) enters slightly acidic early sorting endosomes (EE). Membrane proteins such as the transferrin receptor (TfR) are segregated from soluble content by concentration in fluid-poor tubular extensions and can recycle directly to the membrane (2; fast recycling) or transit rab11-positive recycling endosomes (RE) before returning to the cell surface (3). EEs mature into multivesicular bodies/late endosomes (MVB) by invagination of the limiting membrane that captures ubiquitinated membrane proteins destined for degradation in intralumenal vesicles (4). MVBs release their contents to lysosomes (LY) via transient fusion (5). B: in polarized renal cells, apical and basolateral proteins are internalized into apical and basolateral early endosomes (AEE and BEE; 1a and 1b, respectively). Fast recycling can occur from these compartments (2a and 2b), or membrane proteins, such as the basolaterally recycling TfR, can move into Rab11-negative common recycling endosomes (3a and 3b) before recycling (4a, 4b). At least a subset of apically recycling cargo as well as proteins transcytosing from one membrane to the other also transit the rab11-positive apical recycling endosome before reaching the apical membrane (3a′; 4a). Proteins destined for degradation are targeted deeper into the endocytic pathway from AEE and BEE via formation of MVBs (5) and transient fusion with lysosomes (6). Retrograde transport pathways to the TGN are omitted from A and B for simplicity. C: in addition to clathrin-dependent endocytosis (1), several clathrin independent entry mechanisms have been described (2–5). Some of these pathways, including caveolin and flotillin-dependent internalization (2), as well as a clathrin- and caveolin-independent pathway regulated by RhoA (3), require the GTPase dynamin for fission. A dynamin-independent pathway regulated by ARF6 has also been described (4). GPI-anchored proteins may be internalized into clathrin-independent carriers termed CLICs that fuse with GPI-anchored protein enriched early endosomal compartments (GEECs); whether dynamin is required for CLIC formation remains controversial (5). Interestingly, both clathrin-dependent and -independent pathways ultimately converge in early endosomes, and recycling from this compartment is differentially regulated depending on the mode of entry (see text for more details). In general, is not known whether all of these clathrin independent pathways operate at both apical or basolateral cell surface domains of polarized cells.
Endocytic Pathways in Polarized Cells
Endocytic pathways in polarized cells are by necessity more complex than in nonpolarized cells. The trafficking routes and compartments through which internalized proteins pass have been most extensively characterized in the Madin-Darby canine kidney (MDCK) cell line, which readily forms polarized monolayers when cultured on permeable supports. In these cells, cargo internalized from the apical or basolateral surface is trafficked to distinct apical and basolateral early endosomes, respectively (FIGURE 1B) (20). Apically and basolaterally recycling proteins are trafficked to the common endosome (CRE) before sorting and delivery to the appropriate membrane domain. This compartment is marked by the small GTP binding protein Rab8, although disruption of Rab8 function does not affect recycling (26). The epithelial-specific adaptor protein complex AP1-B, which mediates basolateral recycling of a subset of membrane proteins, is also associated with this endosomal compartment (20). In fully differentiated cells, there is an additional recycling compartment, termed the apical recycling endosome (ARE), that differs from the CRE by the presence of Rab11 and slightly higher pH (6, 88). This compartment, which comprises a subapical cluster of membrane tubules around the microtubule organizing center, has traditionally been thought to receive apically but not basolaterally recycling proteins (2). This view has been recently challenged by studies demonstrating a role of Rab11 and the ARE-associated motor protein Myosin Vb in basolateral recycling (84). Additionally, polarized cells have apical-to-basolateral and basolateral-to-apical transcytotic routes that enable the salvaging of misdirected proteins as well as the bidirectional transit of immunoglobulin receptors. Transcytosing proteins internalized from either cell surface domain transit both the CRE and the ARE before reaching their target membrane (2, 84).
Sorting Decisions Along the Endocytic Pathway
Proteins are sorted at every step along the endocytic pathway. The first of these sorting decisions is whether to enter the pathway at all. Proteins entering the cell via clathrin-dependent endocytosis bind to adaptor proteins that recruit them to clathrin-coated pits (CCPs). Some cell surface receptors engage distinct adaptors for constitutive vs. regulated internalization, resulting ultimately in altered receptor fate. In addition to clathrin-dependent endocytosis, several clathrin-independent mechanisms exist for cargo internalization, and some proteins can access multiple pathways. These pathways converge in early endosomes, where proteins are sorted for recycling, degradation, transcytosis, retrograde transport to the TGN, or sequestration. As discussed below, the fate of a specific protein along this pathway can be acutely modulated by global cues such as cell signaling or by individual posttranslational modifications.
Internalization vs. Cell Surface Retention
Retention factors stabilize the expression of surface membrane proteins, often by preventing or limiting endocytosis. Several retention mechanisms have been described, including association with scaffolding proteins, assimilation into specialized plasmalemma microdomains, and interaction with the cytoskeleton (FIGURE 2A). In recent years, it has become evident that regulation of transport proteins is often achieved by changing the balance between endocytosis and retention. The regulated interplay of retention factors can display an intriguing degree of complexity in these physiological processes.
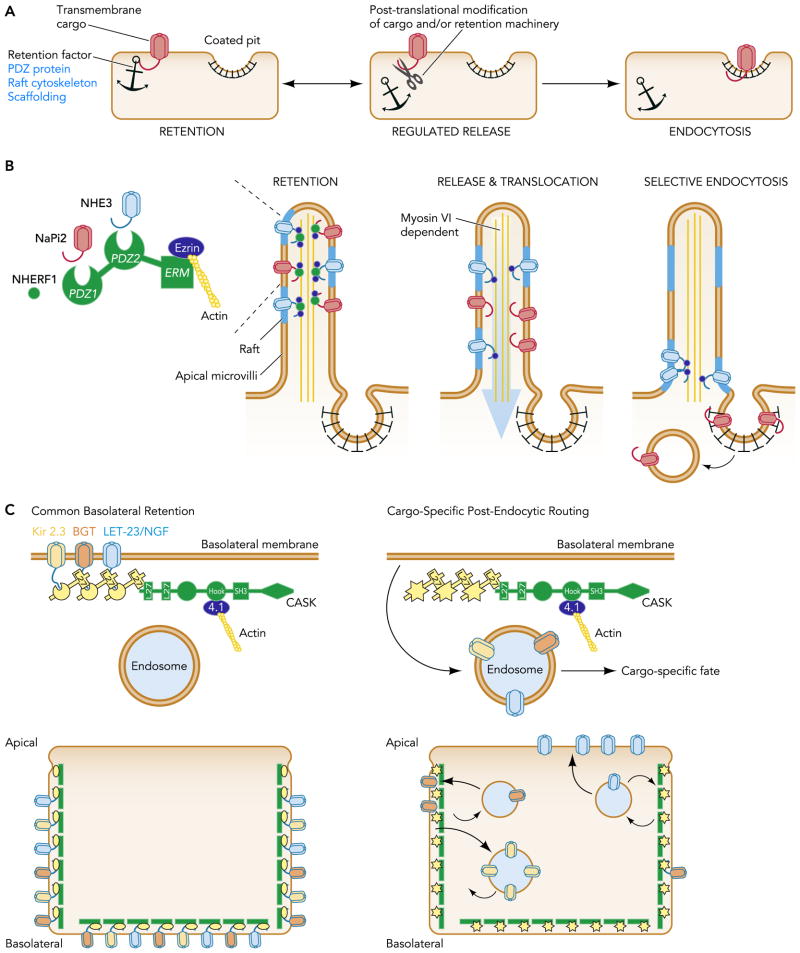
FIGURE 2. Cargo retention mechanisms.
A: General scheme. Retention factors, including PDZ proteins, scaffolding molecules, and the cytoskeleton, can effectively anchor proteins at the plasmalemma and segregate them away from sites of endocytosis. Cargo interactions with retention factors are often tightly controlled by posttranslational modifications. On release from these factors, cargo can be freed for internalization. B: retention of the Na+/H+ exchange isoform 3 (NHE3) and the sodium phosphate cotransporter 2 (NaPi2) at the renal proximal tubule apical membrane. Both transporters associate with the PDZ protein, NHERF1 (Na+/H+ exchange regulatory factor 1), in the apical microvilli, separating them from sites of endocytosis within the intermicrovillar clefts. On release from NHERF1, a myosin VI driven translocation process moves NHE3 and NaPi2 out of the microvilli. Yet, only NaPi2 transporters are internalized. Because NHE3 selectively assimilates with lipid rafts, it is excluded from clathrin-coated pits. C: the Lineage protein 7 (Lin-7) and the calcium-/calmodulin-dependent serine protein kinase (CASK) PDZ protein complex at the basolateral membrane retains a number of PDZ binding targets, including the betaine-GABA transporter (BGT-1), the inwardly rectifying potassium channel Kir 2.3, and the LET-24/NGF receptor tyrosine kinase. Lin-7 directly interacts with cargo. CASK interacts with Lin-7 while simultaneously engaging the actin cytoskeleton through a hook domain interaction with 4.1 proteins. Disruption of Lin-7 interactions produces a wide range of mislocalization phenotypes, depending on the internalization/recycling signals embedded within the cargo proteins.
A salient example is provided by recent discoveries into the mechanisms by which two key sodium transport molecules, the Na+/H+ exchange isoform 3 (NHE3) and the sodium phosphate cotransporter 2 (NaPi2), are physiologically regulated in the renal proximal tubule (FIGURE 2B). NHE3 and NaPi2 are usually localized within the apical microvilli. Retention in this structure segregates the transporters from the sites of avid endocytosis located within the intermicrovillar clefts, allowing robust membrane expression for copious salt reabsorption. Negative regulatory factors, such as PTH, dopamine, or an acute rise in blood pressure, stimulate the translocation of the transporters from the body of the microvilli. But this does not guarantee endocytosis. In fact, only NaPi2 is targeted for internalization (97). NHE3 simply moves to specialized apical membrane domains at the base of the microvilli where its activity is apparently shut off.
Available data suggest the PDZ scaffolding protein, NHERF1 (Na+/H+ exchange regulatory factor 1) (91) and raft-partitioning (67) play separate retention roles in the differential response of the apical sodium-hydrogen exchanger NHE3, and the sodium-phosphate cotransporter NaPi2 to these regulatory signals. NHERF1 effectively anchors NHE3 and NaPi2 within the microvilli by directly interacting with the transporters and simultaneously engaging the underlying microvillar cytoskeleton. NHE3 also directly binds to the actin-associated protein erzin, allowing additional modes of attachment. On signaling through the negative regulatory factors, interactions with NHERF1 become severed, and a myosin VI-driven translocation process moves NHE3 and NaPi2 from the microvilli (4, 98). Because NHE3 selectively assimilates with lipid rafts, the translocated NHE3 molecules are effectively excluded from clathrin-coated pits and consequently retained at the base of the microvilli. By contrast, NaPi2 transporters do not partition into rafts and are free to be internalized once its ties with the microvillar anchor are broken.
It should be pointed out that membrane proteins that interact with the same retention factor may also have different endocytic fates, depending on the internalization/recycling signals embedded within them. A PDZ complex, comprised of Lineage protein 7 (Lin-7) and the calcium-/calmodulin-dependent serine protein kinase (CASK) retains many proteins at the basolateral membrane but disruption of Lin-7 interactions produces a wide range of mislocalization phenotypes (FIGURE 2C). For instance, mutant Kir2.3 channels lacking the PDZ binding motif are largely directed to an endosomal compartment rather than the basolateral membrane (61), consistent with strong endosomal targeting signals. By contrast, mutant betaine-GABA transporters (BGT1) lacking their PDZ binding motif are predominately localized on the basolateral membrane despite increased endocytosis, presumably as a consequence of rapid recycling. In yet another twist on this theme, an apical-missorting phenotype is produced by removing the PDZ binding site from a chimeric LET-23/nerve growth factor receptor protein (78). In this case, Lin-7 interaction may stabilize the receptor on the basolateral membrane to limit transcytosis to the apical membrane.
Cargo Entry Into Clathrin-Coated Pits
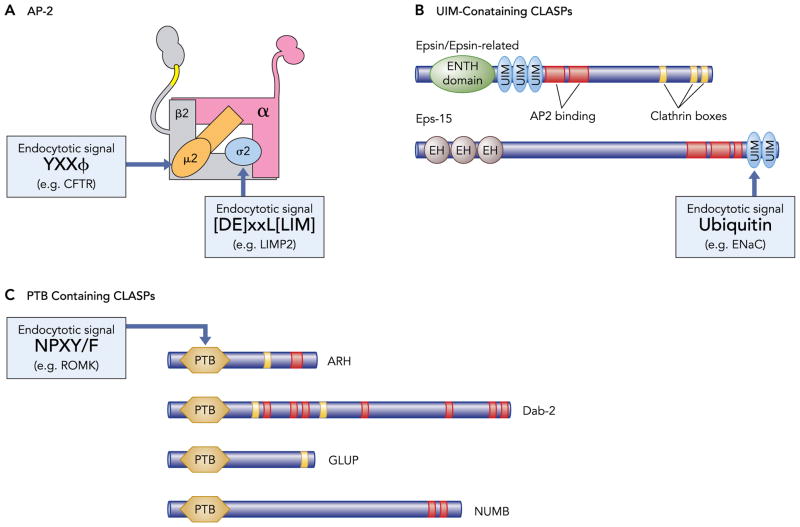
Proteins that are efficiently internalized by clathrin-dependent mechanisms contain cytoplasmic signals that are recognized by intracellular adaptor proteins that link cargo to the forming clathrin lattice (see Ref. 82 for an excellent recent review) (FIGURE 3). The adaptor protein (AP) family is the best-studied class of endocytic adaptor. Individual subunits of the AP-2 adaptor protein complex bind to linear peptide motifs (including YXXΦ and [DE]XXXL[LI] sequences) on the cytoplasmic tail of constitutively internalized cargo proteins. Other adaptor proteins (also termed CLASPs, for clathrin- and lipid-associated sorting proteins) bind variously to different signals on membrane proteins. For instance, one group of CLASPs contain a PTB domain that serves as recognition site for “NPxY/F” endocytosis signals. In some cases, these recognize posttranslational modifications on membrane proteins that target them for regulated internalization; for example, beta-arrestins bind to phosphorylated serine and threonine residues on some activated G-protein-coupled receptors (GPCRs) to modulate their selective internalization and downregulation. Similarly, epidermal growth factor substrate 15 (Eps15), the Eps15-related protein EPS15R, and epsin (for Eps15 interactor) bind to ubiquitin moieties attached to cytoplasmic lysine residues on membrane proteins. The plethora of different CLASPS, each having different signal recognition demands and regulatory requirements, provides a means to tightly control endocytic specificity. For example, clathrin-dependent endocytosis of the epithelial sodium channel (ENaC) and the renal medullary potassium channel (ROMK, Kir1.1) from the principal cell apical membrane can be separately regulated because the channels interact with different CLASPs. ENaC is marked for endocytosis through its interaction with epsin, whereas ROMK is targeted for internalization by the PTB-containing CLASP ARH (FIGURE 3) (18, 89).
FIGURE 3. Mechanisms of adaptor protein interaction with cargo and clathrin.
AP-2 and clathrin- and lipid-associated sorting proteins (CLASPs) have the capacity to simultaneously interact with clathrin (clathrin box) and internalization signals on cargo proteins. Shown are schematic views of the tetrameric AP-2 adaptor protein complex UIM (ubiquitin-interacting motifs)-CLASPS and PTB (phosphotyrosine-binding domain)-containing-CLASPs and their cognate internalization signals. In addition to interacting with clathrin and cargo, the CLASPs also are capable of interacting with AP-2.
In addition to binding clathrin, many CLASPs also interact with AP-2 as well as with other accessory proteins that function in endocytosis. Moreover, nearly all of the CLASPs identified to date bind to the lipid phosphatidylinositol 4,5-bisphosphate (PIP2), which is enriched at the plasma membrane. Formation of this highly scaffolded network of adaptors and accessory proteins facilitates membrane curvature leading to the productive formation of a clathrin-coated vesicle. Fission of the vesicle from the membrane is accomplished by action of the GTPase dynamin.
CLASPs and Cargo Sorting
The availability of numerous CLASPs to engage cargo is one means to ensure access to the endocytic machinery for proteins that are not abundant and/or have relatively weak endocytic sorting signals. Indeed, internalization of a given protein is saturable but does not preclude endocytosis of other proteins with different internalization signals (48, 90). The presence of multiple CLASPs also provides redundancy for specific proteins that has physiological implications: some receptors bind preferentially to one adaptor for constitutive endocytosis but to another on ligand-induced activation. For example the GPCR family member protease-activated receptor-1 (PAR-1) is internalized constitutively by clathrin-mediated endocytosis that requires direct interaction with AP-2. However, ligand binding triggers rapid internalization of PAR-1 via an AP-2-independent pathway (96).
Numerous groups have demonstrated colocalization of multiple receptors or CLASPs in CCPs (8, 10, 35, 49, 80), consistent with the idea that cargo molecules are recruited essentially randomly into coated pits. This notion has been challenged recently by several studies demonstrating the selective enrichment of distinct cargo in subpopulations of clathrin-coated pits (3, 40, 41, 63, 81). In some cases, cargo segregation may be induced on receptor activation. In the case of the epidermal growth factor (EGF) receptor EGF-R, ligand binding has been suggested to lead to de novo assembly of EGF-R-specific CCPs (32, 93), although this idea has been disputed (66). Activated GPCRs also appear to be internalized in a subset of CCPs with unique kinetics (63), and different GPCRs appear to be targeted to distinct subpopulations of preexisting CCPs (54). This segregation may reflect the binding of GPCRs to different adaptor proteins (96). Somewhat surprisingly, however, these GPCRs converge in the same early endosomes after internalization (54). In another study, Lakadamyali et al. found that LDL and EGF are enriched in a subset of CCPs that selectively fuse with a subpopulation of dynamic, rapidly maturing endosomes, whereas the recycling marker transferrin (Tf) is nonselectively delivered to all early endosomes (40).
It is not yet clear whether these disparate observations regarding cargo and adaptor segregation in coated pits can be reconciled with the classical view of coated-pit assembly. Live cell imaging suggests heterogeneity in coated-pit dynamics that could reflect differences in their contents (22), whereas other studies have argued against this idea (16). It is possible that these differences are cell-type specific or that cargo-selective coated pits may form only under experimentally induced conditions. Regardless, it is interesting to speculate about the potential for cargo segregation in polarized cells and the consequent implications for the regulation of renal epithelial transport. There is some evidence for adaptor protein segregation in the most global sense in these cells, since differential recruitment of some CLASPs to one plasma membrane domain or another has been reported. For example, Dab-2 is strikingly positioned at the apical membrane in renal epithelial cells (55), whereas the cellular distribution of ARH in the collecting duct is more diffuse (18). Interestingly, in hepatocytes, ARH is more basolateral, where it is responsible for controlling endocytosis of the LDL receptor. Apical and basolateral CCPs differ in their speed of invagination, with apical endocytosis being markedly slower than basolateral (56). Could these differences explain the presence of CCP populations with distinct lifetimes observed in nonpolarized cells (22)? Are there additional subpopulations of CCPs that might form at each domain?
Clathrin-Independent Endocytosis
In recent years, the contribution of clathrin-independent internalization pathways to cell homeostasis has become increasingly clear. Several distinct clathrin-independent endocytic mechanisms have been identified on the basis of their differential requirement for caveolin, flotillin, dynamin, and the small GTPases Arf 1, Arf6, Rac1, RhoA, and Cdc42. (reviewed in Refs.13, 23, 50, 87; FIGURE 1C). For example, independent caveolar-dependent and RhoA-dependent pathways have been described that also require dynamin, whereas dynamin-independent pathways can be regulated by Cdc42 or Arf6 (50). An important consideration in interpreting studies performed in nonpolarized cells relative to renal physiology is that apical and basolateral surfaces differ in the availability of clathrin-independent mechanisms as alternative endocytic pathways. For example, the apical surface of polarized renal cells has been reported to lack caveolae (86), although caveolin-1, a component of caveolae, is apparently present at the apical surface of MDCK cells and apical transporters such as UT-A1 physically interact with this protein in lipid rafts (19, 71). The relative robustness of these individual pathways in differentiated plasma membrane domains vs. undifferentiated cells may explain why urokinase plasminogen activator receptor utilizes distinct internalization pathways in polarized vs. nonpolarized MDCK cells (87).
Proteins internalized by clathrin-independent pathways are initially observed in PI3P-negative compartments, but many converge with clathrin-dependent cargo in Rab5 and PI3P-positive EEs (14, 58). An exception may be proteins that are attached to the membrane via glycosylphosphatidylinositol (GPI) anchors. At least some of these proteins are internalized into specialized endocytic compartments termed GEECs (for GPI-anchored-protein-enriched endosomal compartments) or CLICs (for clathrin-independent carriers) that are also enriched in fluid phase markers. GEECs/CLICs require Cdc42 and the GTPase activating protein GRAF1 for formation, and in some studies GPI-anchored proteins internalized in these compartments remain segregated from early endosomes containing internalized Tf (57, 70). Whether dynamin plays a role in the GEEC/CLIC pathway is controversial (46, 70).
Some proteins can utilize multiple endocytic mechanisms to enter cells, sometimes with distinct consequences to their fate. Cholera toxin internalization via its receptor (the ganglioside GM1) occurs via both clathrin-dependent and GEEC/CLIC pathways (37, 59). In the case of the EGF-R, internalization via the clathrin-dependent pathway is important for signaling but leads to less efficient degradation compared with internalization by a clathrin-independent pathway (76). In some cells, EGF-R internalization via a clathrin-independent pathway at high ligand concentrations may reflect saturation of the clathrin-mediated pathway (94). Neither of these pathways appears to absolutely require ubiquitination of EGF-R, although this modification is essential for targeting the receptor to lysosomes (29). A more comprehensive discussion of EGF-R trafficking can be found in Ref. 77. In contrast, internalization of the potassium channel ROMK may be diverted from a clathrin-dependent pathway to a clathrin- and dynamin-independent pathway on ubiquitination by the ubiquitin ligase POSH (44). A caveat to consider in interpreting such studies is that experimental perturbation of endocytic pathways may lead to compensatory endocytosis of cargo via alternative mechanisms (12, 70).
Protein Recycling
Internalized proteins destined for delivery to the cell surface can undergo slow or fast recycling. “Fast” recycling occurs from early sorting endosomes or earlier compartments, and this process is regulated by Rab4 and Rab35 (39, 85). In contrast, “slow” recycling in nonpolarized cells involves cargo passage through Rab11-positive recycling endosomes, which are formed from membrane-rich tubules that emanate from sorting endosomes. In these cells, disrupting the function of proteins important for transport to recycling endosomes, such as Rab11 effectors, actually enhances recycling of proteins internalized via clathrin-dependent mechanisms, probably via the fast recycling pathway (72).
Recent data suggest that proteins can segregate into distinct subdomains of recycling endosomes and point to the existence of multiple pathways for recycling. Oddly, it appears from several studies that recycling of clathrin-dependent and clathrin-independent cargo from recycling endosomes proceeds via distinct carriers and is differentially regulated by Rabs (reviewed in Ref. 23). Whereas recycling of plasma membrane proteins internalized via clathrin-dependent mechanisms appears to represent the default pathway for cargo handling, recycling of clathrin-independent proteins is highly regulated. A recent review by Julie Donaldson points out that several proteins regulate both clathrin-dependent endocytosis and clathrin-independent recycling, and suggests that this redundancy may enable coordination of both pathways (14).
A subset of proteins apparently have specific targeting information that regulates their exit from recycling endosomes. Expression of a dominant-negative form of Rme-1, a protein that regulates cargo exit from recycling endosomes, in HEK 293 cells results in accumulation of Tf receptor (TfR) and the cystic fibrosis transmembrane conductance regulator (CFTR) in recycling endosomes, whereas trafficking of low-density lipoprotein is unaffected (62).
Recycling of a given protein can be affected individually by cell signaling events. In some cases, ligand binding can influence the fate of a receptor: for example, internalized EGF-R bound to EGF is targeted to lysosomes for degradation, whereas the alternative ligand TGF-α is released from the receptor in acidic EEs, resulting in efficient EGF-R recycling to the plasma membrane (21). This postendocytic routing may reflect the use of distinct internalization pathways for different EGF-R-ligand complexes.
There are also cases where cell signaling causes global alterations in protein traffic. For example, activation of the ephrin B receptor in rat hippocampal neurons causes phosphorylation of synaptojanin 1, which decreases its activity and globally stimulates internalization of TfR and other receptors (31). Similarly, ligand-induced endocytosis of the beta-2 adrenergic receptor in HEK293 cells stimulates a Rab4-dependent recycling pathway that also increases recycling of TfR (99). Alternatively, some signaling pathways can globally inhibit protein recycling, since sustained GPCR signaling by serotonin led to sequestration of the 5-HT receptor as well as other proteins (EGF-R and PAR-1) in intracellular compartments of HEK293 cells (30).
Regulation of Cargo Trafficking by Posttranslational Modifications
In recent years, intense investigation into elucidating the mechanisms by which internalization and postendocytic traffic of cargo molecules may be physiologically controlled has illuminated key roles of phosphorylation, ubiquitination, and palmitoylation.
Modulation of Endocytosis by Phosphorylation
Phosphorylation-dependent regulation of endocytic trafficking has been implicated in a wide range of physiological processes from synaptic transmission to the regulation of salt and mineral balance. Elucidating how this occurs has been the focus of intense investigation. Following the discovery that rapid endocytosis of the polymeric immunoglobulin receptor from the MDCK cell basolateral membrane requires phosphorylation of a cytoplasmic serine residue (60), many epithelial transport molecules have been found to be internalized or routed in the post-endocyotic pathway by processes that are dependent on their own phosphorylation status. For some, such as the apical Na+/H+ exchanger NHE3 (28) and the vasopressin-regulated water channel aquaporin 2 (AQP2) (53), serine or threonine phosphorylation is required. For others, including the ROMK potassium secretory channel, tyrosine phosphorylation can play a role (42).
Examples are not limited to proteins, like those above, that are internalized by clathrin-dependent endocytosis. Raft-mediated endocytosis of nephrin, for instance, is triggered by tyrosine phosphorylation, in a response that has been implicated in the assembly of the glomerular slit diaphragm (64). Phosphorylation can also block entry of cargo into clathrin-independent internalization vehicles. For instance, in HEK 293 cells, phosphorylation of the apical TRPV5 channel suppresses endocytosis into caveolae. This response has been suggested to explain how Ca2+ reabsorption in the distal nephron may be stimulated by parathyroid-hormone (9).
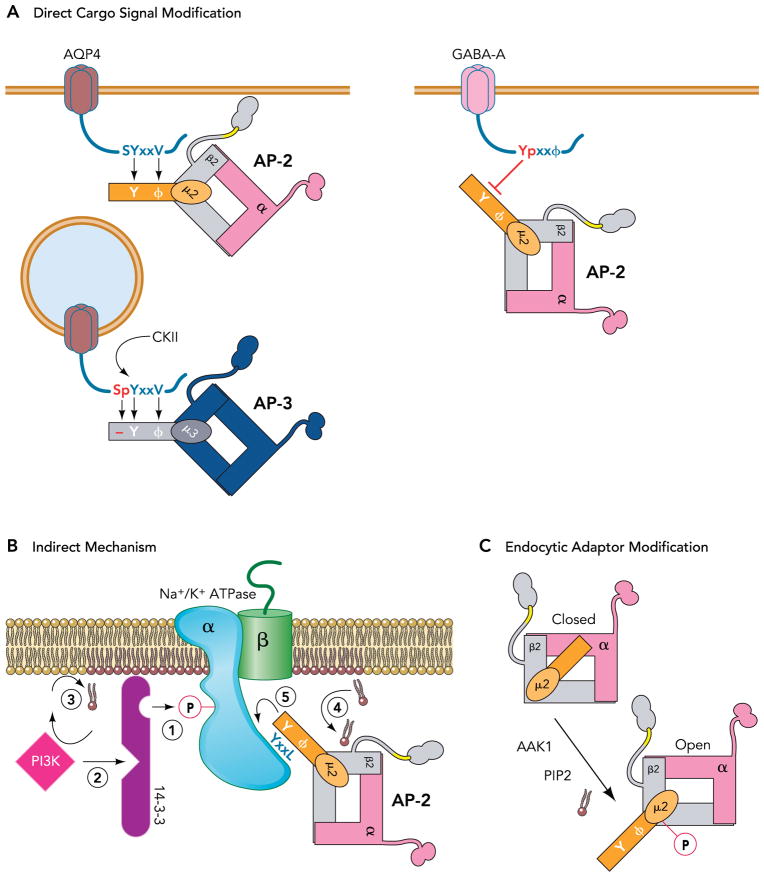
Many different mechanisms have been implicated in translating a cargo phosphorylation event into an endocytic trafficking command (FIGURE 4). As exemplified by the interaction of GPCRs with arrestins, phosphorylation can create a recognition site for internalization machinery de novo. In other cases, phosphorylation can refine an existing endocytic trafficking signal so as to optimize or inhibit cargo interaction with specific components of the endocytic machinery. With aquaporin 4 water channels, for example, phosphorylation of a serine immediately preceding the YXXΦ-adaptin interaction motif switches the specificity of the clathrin adaptor recognition site from AP-2 to the lysosome targeting adaptor protein complex AP-3. This directs phosphorylated channels for traffic into the degradative pathway (47). By contrast, phosphorylation of the key tyrosine in the GABA-“A” receptor’s “YXXΦ” internalization motif renders the signal unable to engage the AP-2 adaptor protein complex (38), suppressing endocytosis and up-regulating surface levels of the receptor for synaptic inhibition.
FIGURE 4. Mechanisms by which endocytosis is controlled by phosphorylation.
A: phosphorylation of residues within or near internalization signals can enhance (AQP4) or inhibit (GABA-A receptor) their interaction with endocytotic machinery. B: in transport proteins, like the Na-K-ATPase (see text), phosphorylation at sites other than the endocytotic signal can create interaction sites for proteins that are indirectly involved in endocytosis. C: endocytotic machinery is also regulated by phosphorylation. For example, the binding site for tyrosine-based internalization signals on the AP-2 complex (mu subunit) is exposed on phosphorylation by AAK1 (or by PIP2 association).
More commonly described mechanisms involve phosphorylation of sites that seem far removed from endocytic motifs. In many of these cases, it has been speculated that phosphorylation may change cargo protein conformation in such a way to affect presentation or accessibility of the internalization signal. Sometimes, however, phosphorylation can create or modify important docking sites for proteins that are indirectly involved in endocytosis. This is just how endocytosis of the Na+-K+-ATPase may be stimulated by dopamine (11). On activation of the dopamine receptor, PKC-mediated phosphorylation of a serine residue in the pump’s catalytic alpha-1 subunit produces a binding site for 14-3-3 proteins (15). Recruitment of 14-3-3 to the Na+-K+-ATPase facilitates subsequent binding of PI-3 kinase, and this, in turn, stimulates endocytosis, likely by changing the polyphosphoinositide chemistry around the Na+/K+ pump. An increase in the local pool of PIP2, for example, stimulates cargo endocytosis because PIP2 increases the binding affinity of the AP-2 for tyrosine and di-leucine-based endocytic signals (27).
In other cases, phosphorylation abrogates cargo interaction with retention proteins, freeing cargo for endocytosis. An important example is provided by type I PDZ protein interactions. Phosphorylation of the key serine or threonine residue in the cargo PDZ binding site is well appreciated to create an energetically unfavorable substrate for PDZ retention protein interaction.
Phosphorylation can be modulated by the regulated targeting of kinases and other signaling molecules to sites of endocytosis or to endosomes, providing a means to adjust the internalization and post-endocytic traffic of cargo molecules with physiological needs. Such a mechanism has been proposed to influence how the With No Lysine (WNKs) kinases WNK1 and WNK4 control renal salt transport. These kinases normally control the balance between renal sodium and potassium excretion by regulating the thiazide-sensitive sodium chloride cotransporter, NCC, and the ROMK potassium channel in opposite directions. In cell expression models, both kinases stimulate clathrin-dependent endocytosis of ROMK (18, 33), but neither WNK kinase influences NCC internalization. Instead, WNK4 stimulates interaction of NCC with the clathrin adaptor AP-3 and sortilin in the secretory pathway (79, 101), so as to target newly synthesized transporters to lysosomes. Because lysosomal targeting of NCC is suppressed by WNK1, physiological changes in the relative abundance of WNK1 and WNK4 kinase may help switch the signaling pathway to either block or permit NCC surface delivery.
Targeting of the WNK kinases to different endosomal locales may toggle the signaling pathway to preferentially stimulate ROMK endocytosis or activate NCC lysosome routing. WNK1 and WNK4 are normally recruited to clathrin-coated pits through their interaction with the multimodular endocytic scaffold intersectin (25). However, WNK1 can translocate to the TGN and/or recycling endosomes on phosphorylation and exposure to hypotonic stress (100). This targeting is apparently faulty in mutations of WNK1 and WNK4 that cause pseudohypoaldosteronism type II (PHAII), a familial disorder of renal potassium retention and hypertension in which increased NCC and decreased ROMK at the apical surface result in exaggerated sodium reabsorption and diminished potassium urinary excretion (95). PHAII-causing mutations in WNK4 increase the affinity of the kinase for intersectin and thereby augment ROMK endocytosis in the disease. Sequestering the kinase to the sites of internalization at the cell surface would also diminish the amount of kinase available to stimulate lysosomal trafficking of NCC, offering a potential explanation for the pathophysiological increase in NCC at the cell surface.
In addition to the above examples where trafficking of individual cargo can be modulated by phosphorylation, it should also be pointed out that the endocytic machinery itself is tightly regulated by various kinases. Cycles of AP-2 phosphorylation and dephosphorylation by the adaptor-associated kinase 1 (AAK1) have been proposed to orchestrate cycles of cargo recruitment to clathrin-coated pits and AP-2 uncoating from early endocytic vesicles (74), and internalization of caveolae also requires phosphorylation of the endocytic machinery, likely involving Src-mediated tyrosine phosphorylation of caveolin-1 (52).
Ubiquitination-Dependent Protein Trafficking
The posttranslational attachment ubiquitin can serve as a signal to target certain transmembrane proteins for internalization. Accumulating evidence indicates the addition of multiple monoubiquitin moieties per cargo molecule (multiple monoubiquitination) or the addition of polyubiquitin chains are usually involved, rather that the addition of a single ubiquitin (monoubiquitination) as once believed (reviewed in Ref. 83). In fact, for some proteins, like AQP2 (34), the conjugation of more than one ubiquitin seems to be absolutely required for endosomal trafficking. For others, like ENaC, the ubiquitin chain might behave as an endocytic rheostat, increasing the efficiency of internalization with growing length (92, 102).
Ubiquitination not only marks surface proteins for endocytosis, it also can target internalized proteins for traffic to MVBs and lysosomes. Two components of the endosomal sorting complexes required for transport (ESCRT) machinery (65), called HRS (hepatocyte growth factor regulated tyrosine kinase substrate) and STAM (signal transducing adaptor molecule), are responsible for capturing internalized, ubiquitated cargo in the early endosome for inclusion in MVBs. Because HRS and STAM also initiate the recruitment of the ESCRT machineries that are responsible for forming the intraluminal vesicles of the MVB, they essentially couple cargo-selection to MVB genesis.
In recent years, it has become evident that ubiquitination drives the endocytic traffic of many epithelial transport molecules, including ENaC (92, 102), CFTR (5, 75), ROMK (43, 44), AQP2 (34), and the ClC-5 H+/Cl− antiporter (73). In fact, the regulated addition and removal of ubiquitin affords an important means to tightly control the surface density of these epithelial transport molecules. Ex-emplified by the modulation of ENaC by Nedd4 (69), the association of E3 ubiquitin ligases with their substrates is usually highly specific and precisely regulated, allowing endocytosis and/or lysosomal routing of specific cargo proteins to be selectively stimulated in accord with physiological needs. An equally important, but less studied, mechanism for turning off endosomal routing involves the removal of ubiquitin from cargo proteins by deubquitinating enzymes (DUBs). Like E3 ligases, DUB specificity is determined by substrate-specific binding and by specific subcellular compartmentalization. For example, UCH-L3 (7) and USP10 (5) localize to populations of early endosomal to deubiquitinate internalized cargoes, including ENaC and CFTR. Also similar to the E3 ligases, the activity of DUBs can be tightly regulated. UCH-L3, for example, is apparently stimulated by cAMP/PKA so as to enhance ENaC recycling. Another DUB that deubiquitinates ENaC, USP2–45, is induced by aldosterone, providing a means to physiologically increase ENaC at the cell surface in hyperaldosterone states (17).
Importantly, alterations in ubiquitin-dependent sorting processes can give rise to serious human diseases of altered fluid and electrolyte balance. For example, mutations in ENaC that corrupt the “PPXY” binding site for Nedd4 render the channel unable to be efficiently cleared from the apical membrane. This leads to Liddle’s syndrome, an inherited disease of excessive sodium reabsorption and hypertension. General misfolding appears to exacerbate ubiquitin-dependent routing of mutant CFTR molecules to the lysosome, contributing to defective Cl− transport in cystic fibrosis (75).
Palmitoylation
Once believed to operate singularly as a membrane anchor for cementing cytoplasmic proteins to membranes, accumulating evidence now indicates that thioester linkage of palmitate, a C16 saturated fatty acid, to internal (S-palmitoylation) or NH2-terminal cysteines (N-palmitylation) does much more. For a growing number of integral membrane proteins, palmitoylation can generate trafficking cues. Awareness of palmitoylation as an endocytic signal recently became particularly heightened with the realization that it plays an important role in the internalization of the Anthrax toxin receptor (1).
As it turns out, palmitoylation can drive a variety of different trafficking commands besides endocytosis. Depending on the target molecule, it can control biosynthetic delivery, surface membrane retention, or transport along the post-endocytic pathway. Palmitoylation of the apical membrane transmembrane mucin MUC1 drives recycling from endosomes back to the plasma membrane (36). However, in the case of the lysosomal enzyme receptors sortilin and the mannose 6-phosphate receptor, palmitoylation influences recycling back to the Golgi, likely by facilitating the translocation of receptors to a membrane compartment that contains retromer (51). Palmitoylation of NMDA can either stimulate surface membrane retention or inhibit Golgi export, depending on where on the protein palmitate is added (24). Because S-palmitoylation is reversible, cycles of palmitate addition and removal may affect trafficking of the same target protein at several different steps in its travels to and from the cell surface. With recent discoveries of the protein acyltransferase enzyme families that catalyze the addition of palmitate (45) and the development of new techniques to identify palmitoylated proteins on a proteomic scale (68), the field appears to be on the verge of solving the mystery of how one type of modification can control so many diverse trafficking processes.
Summary
Endocytic pathways and their regulation in polarized cells is more complex than in non-polarized cells, and results obtained using nonpolarized cells do not necessarily translate to the situation in polarized cells. As examples, clathrin-dependent apical endocytosis is markedly slower than basolateral endocytosis, and caveolae have been reported to be absent from the apical surface.
Individual steps along the endocytic pathway are regulated independently to modulate the surface density of a given membrane protein. Sorting decisions include internalization vs. cell surface retention, the endocytic pathway engaged, and the subsequent fate of the internalized protein (e.g., intracellular retention, recycling, or degradation).
Clathrin-dependent endocytosis of proteins can be differentially regulated via their interactions with distinct adaptors. There is increasing evidence for the existence of subsets of clathrin-coated pits that are enriched in selected cargo proteins. Some receptors engage different CLASPs during constitutive vs. ligand-induced endocytosis.
There are multiple pathways for membrane recycling. Fast recycling bypasses recycling endosomes entirely. Export from recycling endosomes of proteins internalized by clathrin-dependent vs. -independent mechanisms appears to be differentially regulated. Cell signaling can alter the endocytosis and/or recycling of individual proteins or globally affect membrane traffic.
Trafficking of individual cargo can be modulated by many reversible posttranslational modifications, including phosphorylation, ubiquitination/deubiquitination, and palmitoylation.
Acknowledgments
We thank John Johnson and Alexander Sorkin for thoughtful comments.
Studies on protein traffic in the authors’ laboratories are supported by National Institute of Diabetes and Digestive and Kidney Diseases R01 Grants DK-54407 and DK-064613 (to O. A. Weisz), and DK-54281 and DK-63049 (to P. A. Welling).
Footnotes
No conflicts of interest, financial or otherwise, are declared by the author(s).
References
- 1.Abrami L, Leppla SH, van der Goot FG. Receptor palmitoylation and ubiquitination regulate anthrax toxin endocytosis. J Cell Biol. 2006;172:309–320. doi: 10.1083/jcb.200507067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Apodaca G, Katz LA, Mostov KE. Receptor-mediated transcytosis of IgA in MDCK cells is via apical recycling endosomes. J Cell Biol. 1994;125:67–86. doi: 10.1083/jcb.125.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benmerah A, Lamaze C. Clathrin-coated pits: vive la difference? Traffic. 2007;8:970–982. doi: 10.1111/j.1600-0854.2007.00585.x. [DOI] [PubMed] [Google Scholar]
- 4.Blaine J, Okamura K, Giral H, Breusegem S, Caldas Y, Millard A, Barry N, Levi M. PTH-induced internalization of apical membrane NaPi2a: role of actin and myosin VI. Am J Physiol Cell Physiol. 2009;297:C1339–C1346. doi: 10.1152/ajpcell.00260.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bomberger JM, Barnaby RL, Stanton BA. The deubiquitinating enzyme USP10 regulates the post-endocytic sorting of cystic fibrosis transmembrane conductance regulator in airway epithelial cells. J Biol Chem. 2009;284:18778–18789. doi: 10.1074/jbc.M109.001685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown PS, Wang E, Aroeti B, Chapin SJ, Mostov KE, Dunn KW. Definition of distinct compartments in polarized Madin-Darby canine kidney (MDCK) cells for membrane-volume sorting, polarized sorting and apical recycling. Traffic. 2000;1:124–140. doi: 10.1034/j.1600-0854.2000.010205.x. [DOI] [PubMed] [Google Scholar]
- 7.Butterworth MB, Edinger RS, Ovaa H, Burg D, Johnson JP, Frizzell RA. The deubiquitinating enzyme UCH-L3 regulates the apical membrane recycling of the epithelial sodium channel. J Biol Chem. 2007;282:37885–37893. doi: 10.1074/jbc.M707989200. [DOI] [PubMed] [Google Scholar]
- 8.Carpentier JL, Gorden P, Anderson RG, Goldstein JL, Brown MS, Cohen S, Orci L. Co-localization of 125I-epidermal growth factor and ferritin-low density lipoprotein in coated pits: a quantitative electron microscopic study in normal and mutant human fibroblasts. J Cell Biol. 1982;95:73–77. doi: 10.1083/jcb.95.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cha SK, Wu T, Huang CL. Protein kinase C inhibits caveolae-mediated endocytosis of TRPV5. Am J Physiol Renal Physiol. 2008;294:F1212–F1221. doi: 10.1152/ajprenal.00007.2008. [DOI] [PubMed] [Google Scholar]
- 10.Chen H, Fre S, Slepnev VI, Capua MR, Takei K, Butler MH, Di Fiore PP, De Camilli P. Epsin is an EH-domain-binding protein implicated in clathrin-mediated endocytosis. Nature. 1998;394:793–797. doi: 10.1038/29555. [DOI] [PubMed] [Google Scholar]
- 11.Chibalin AV, Pedemonte CH, Katz AI, Feraille E, Berggren PO, Bertorello AM. Phosphorylation of the catalyic alpha-subunit constitutes a triggering signal for Na+, K+-ATPase endocytosis. J Biol Chem. 1998;273:8814–8819. doi: 10.1074/jbc.273.15.8814. [DOI] [PubMed] [Google Scholar]
- 12.Damke H, Baba T, van der Bliek AM, Schmid SL. Clathrin-independent pinocytosis is induced in cells overexpressing a temperature-sensitive mutant of dynamin. J Cell Biol. 1995;131:69–80. doi: 10.1083/jcb.131.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doherty GJ, McMahon HT. Mechanisms of endocytosis. Annu Rev Biochem. 2009;78:857–902. doi: 10.1146/annurev.biochem.78.081307.110540. [DOI] [PubMed] [Google Scholar]
- 14.Donaldson JG, Porat-Shliom N, Cohen LA. Clathrin-independent endocytosis: a unique platform for cell signaling and PM remodeling. Cell Signal. 2009;21:1–6. doi: 10.1016/j.cellsig.2008.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Efendiev R, Chen Z, Krmar RT, Uhles S, Katz AI, Pedemonte CH, Bertorello AM. The 14-3-3 protein translates the Na+, K+-ATPase α1-subunit phosphorylation signal into binding and activation of phosphoinositide 3-kinase during endocytosis. J Biol Chem. 2005;280:16272–16277. doi: 10.1074/jbc.M500486200. [DOI] [PubMed] [Google Scholar]
- 16.Ehrlich M, Boll W, Van Oijen A, Hariharan R, Chandran K, Nibert ML, Kirchhausen T. Endocytosis by random initiation and stabilization of clathrin-coated pits. Cell. 2004;118:591–605. doi: 10.1016/j.cell.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 17.Fakitsas P, Adam G, Daidie D, van Bemmelen MX, Fouladkou F, Patrignani A, Wagner U, Warth R, Camargo SM, Staub O, Verrey F. Early aldosterone-induced gene product regulates the epithelial sodium channel by deubiquitylation. J Am Soc Nephrol. 2007;18:1084–1092. doi: 10.1681/ASN.2006080902. [DOI] [PubMed] [Google Scholar]
- 18.Fang L, Garuti R, Kim BY, Wade JB, Welling PA. The ARH adaptor protein regulates endocytosis of the ROMK potassium secretory channel in mouse kidney. J Clin Invest. 2009;119:3278–3289. doi: 10.1172/JCI37950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feng X, Huang H, Yang Y, Frohlich O, Klein JD, Sands JM, Chen G. Caveolin-1 directly interacts with UT-A1 urea transporter: the role of caveolae/lipid rafts in UT-A1 regulation at the cell membrane. Am J Physiol Renal Physiol. 2009;296:F1514–F1520. doi: 10.1152/ajprenal.00068.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Folsch H, Mattila PE, Weisz OA. Taking the scenic route: biosynthetic traffic to the plasma membrane in polarized epithelial cells. Traffic. 2009;10:972–981. doi: 10.1111/j.1600-0854.2009.00927.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.French AR, Tadaki DK, Niyogi SK, Lauffenburger DA. Intracellular trafficking of epidermal growth factor family ligands is directly influenced by the pH sensitivity of the receptor/ligand interaction. J Biol Chem. 1995;270:4334–4340. doi: 10.1074/jbc.270.9.4334. [DOI] [PubMed] [Google Scholar]
- 22.Gaidarov I, Santini F, Warren RA, Keen JH. Spatial control of coated-pit dynamics in living cells. Nat Cell Biol. 1999;1:1–7. doi: 10.1038/8971. [DOI] [PubMed] [Google Scholar]
- 23.Grant BD, Donaldson JG. Pathways and mechanisms of endocytic recycling. Nat Rev Mol Cell Biol. 2009;10:597–608. doi: 10.1038/nrm2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hayashi T, Thomas GM, Huganir RL. Dual palmitoylation of NR2 subunits regulates NMDA receptor trafficking. Neuron. 2009;64:213–226. doi: 10.1016/j.neuron.2009.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.He G, Wang HR, Huang SK, Huang CL. Intersectin links WNK kinases to endocytosis of ROMK1. J Clin Invest. 2007;117:1078–1087. doi: 10.1172/JCI30087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Henry L, Sheff DR. Rab8 regulates basolateral secretory, but not recycling, traffic at the recycling endosome. Mol Biol Cell. 2008;19:2059–2068. doi: 10.1091/mbc.E07-09-0902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Honing S, Ricotta D, Krauss M, Spate K, Spolaore B, Motley A, Robinson M, Robinson C, Haucke V, Owen DJ. Phosphatidylinositol-(4,5)-bisphosphate regulates sorting signal recognition by the clathrin-associated adaptor complex AP2. Mol Cell. 2005;18:519–531. doi: 10.1016/j.molcel.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 28.Hu MC, Fan L, Crowder LA, Karim-Jimenez Z, Murer H, Moe OW. Dopamine acutely stimulates Na+/H+ exchanger (NHE3) endocytosis via clathrin-coated vesicles: dependence on protein kinase A-mediated NHE3 phosphorylation. J Biol Chem. 2001;276:26906–26915. doi: 10.1074/jbc.M011338200. [DOI] [PubMed] [Google Scholar]
- 29.Huang F, Goh LK, Sorkin A. EGF receptor ubiquitination is not necessary for its internalization. Proc Natl Acad Sci USA. 2007;104:16904–16909. doi: 10.1073/pnas.0707416104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Idkowiak-Baldys J, Baldys A, Raymond JR, Hannun YA. Sustained receptor stimulation leads to sequestration of recycling endosomes in a classical protein kinase C- and phospholipase D-dependent manner. J Biol Chem. 2009;284:22322–22331. doi: 10.1074/jbc.M109.026765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Irie F, Okuno M, Pasquale EB, Yamaguchi Y. EphrinB-EphB signalling regulates clathrin-mediated endocytosis through tyrosine phosphorylation of synaptojanin 1. Nat Cell Biol. 2005;7:501–509. doi: 10.1038/ncb1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johannessen LE, Pedersen NM, Pedersen KW, Madshus IH, Stang E. Activation of the epidermal growth factor (EGF) receptor induces formation of EGF receptor- and Grb2-containing clathrin-coated pits. Mol Cell Biol. 2006;26:389–401. doi: 10.1128/MCB.26.2.389-401.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kahle KT, Wilson FH, Leng Q, Lalioti MD, O’Connell AD, Dong K, Rapson AK, MacGregor GG, Giebisch G, Hebert SC, Lifton RP. WNK4 regulates the balance between renal NaCl reabsorption and K+ secretion. Nat Genet. 2003;35:372–376. doi: 10.1038/ng1271. [DOI] [PubMed] [Google Scholar]
- 34.Kamsteeg EJ, Hendriks G, Boone M, Konings IB, Oorschot V, van der Sluijs P, Klumperman J, Deen PM. Short-chain ubiquitination mediates the regulated endocytosis of the aquaporin-2 water channel. Proc Natl Acad Sci USA. 2006;103:18344–18349. doi: 10.1073/pnas.0604073103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Keyel PA, Mishra SK, Roth R, Heuser JE, Watkins SC, Traub LM. A single common portal for clathrin-mediated endocytosis of distinct cargo governed by cargo-selective adaptors. Mol Biol Cell. 2006;17:4300–4317. doi: 10.1091/mbc.E06-05-0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kinlough CL, McMahan RJ, Poland PA, Bruns JB, Harkleroad KL, Stremple RJ, Kashlan OB, Weixel KM, Weisz OA, Hughey RP. Recycling of MUC1 is dependent on its palmitoylation. J Biol Chem. 2006;281:12112–12122. doi: 10.1074/jbc.M512996200. [DOI] [PubMed] [Google Scholar]
- 37.Kirkham M, Fujita A, Chadda R, Nixon SJ, Kurzchalia TV, Sharma DK, Pagano RE, Hancock JF, Mayor S, Parton RG. Ultrastructural identification of uncoated caveolin-independent early endocytic vehicles. J Cell Biol. 2005;168:465–476. doi: 10.1083/jcb.200407078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kittler JT, Chen G, Kukhtina V, Vahedi-Faridi A, Gu Z, Tretter V, Smith KR, McAinsh K, Arancibia-Carcamo IL, Saenger W, Haucke V, Yan Z, Moss SJ. Regulation of synaptic inhibition by phospho-dependent binding of the AP2 complex to a YECL motif in the GABAA receptor gamma2 subunit. Proc Natl Acad Sci USA. 2008;105:3616–3621. doi: 10.1073/pnas.0707920105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kouranti I, Sachse M, Arouche N, Goud B, Echard A. Rab35 regulates an endocytic recycling pathway essential for the terminal steps of cytokinesis. Curr Biol. 2006;16:1719–1725. doi: 10.1016/j.cub.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 40.Lakadamyali M, Rust MJ, Zhuang X. Ligands for clathrin-mediated endocytosis are differentially sorted into distinct populations of early endosomes. Cell. 2006;124:997–1009. doi: 10.1016/j.cell.2005.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leonard D, Hayakawa A, Lawe D, Lambright D, Bellve KD, Standley C, Lifshitz LM, Fogarty KE, Corvera S. Sorting of EGF and transferrin at the plasma membrane and by cargo-specific signaling to EEA1-enriched endosomes. J Cell Sci. 2008;121:3445–3458. doi: 10.1242/jcs.031484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lin DH, Sterling H, Lerea KM, Welling P, Jin L, Giebisch G, Wang WH. K depletion increases protein tyrosine kinase-mediated phosphorylation of ROMK. Am J Physiol Renal Physiol. 2002;283:F671–F677. doi: 10.1152/ajprenal.00160.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lin DH, Sterling H, Wang Z, Babilonia E, Yang B, Dong K, Hebert SC, Giebisch G, Wang WH. ROMK1 channel activity is regulated by monoubiquitination. Proc Natl Acad Sci USA. 2005;102:4306–4311. doi: 10.1073/pnas.0409767102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lin DH, Yue P, Pan CY, Sun P, Zhang X, Han Z, Roos M, Caplan M, Giebisch G, Wang WH. POSH stimulates the ubiquitination and the clathrin-independent endocytosis of ROMK1 channels. J Biol Chem. 2009;284:29614–29624. doi: 10.1074/jbc.M109.041582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Linder ME, Deschenes RJ. Palmitoylation: policing protein stability and traffic. Nat Rev Mol Cell Biol. 2007;8:74–84. doi: 10.1038/nrm2084. [DOI] [PubMed] [Google Scholar]
- 46.Lundmark R, Doherty GJ, Howes MT, Cortese K, Vallis Y, Parton RG, McMahon HT. The GTPase-activating protein GRAF1 regulates the CLIC/GEEC endocytic pathway. Curr Biol. 2008;18:1802–1808. doi: 10.1016/j.cub.2008.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Madrid R, Le Maout S, Barrault MB, Janvier K, Benichou S, Merot J. Polarized trafficking and surface expression of the AQP4 water channel are coordinated by serial and regulated interactions with different clathrin-adaptor complexes. EMBO J. 2001;20:7008–7721. doi: 10.1093/emboj/20.24.7008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marks MS, Woodruff L, Ohno H, Bonifacino JS. Protein targeting by tyrosine- and di-leucine-based signals: evidence for distinct saturable components. J Cell Biol. 1996;135:341–354. doi: 10.1083/jcb.135.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maxfield FR, Schlessinger J, Shechter Y, Pastan I, Willingham MC. Collection of insulin, EGF and alpha2-macroglobulin in the same patches on the surface of cultured fibroblasts and common internalization. Cell. 1978;14:805–810. doi: 10.1016/0092-8674(78)90336-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mayor S, Pagano RE. Pathways of clathrin-independent endocytosis. Nat Rev Mol Cell Biol. 2007;8:603–612. doi: 10.1038/nrm2216. [DOI] [PubMed] [Google Scholar]
- 51.McCormick PJ, Dumaresq-Doiron K, Pluviose AS, Pichette V, Tosato G, Lefrancois S. Palmitoylation controls recycling in lysosomal sorting and trafficking. Traffic. 2008;9:1984–1997. doi: 10.1111/j.1600-0854.2008.00814.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Minshall RD, Tiruppathi C, Vogel SM, Niles WD, Gilchrist A, Hamm HE, Malik AB. Endothelial cell-surface gp60 activates vesicle formation and trafficking via Gi-coupled Src kinase signaling pathway. J Cell Biol. 2000;150:1057–1070. doi: 10.1083/jcb.150.5.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moeller HB, Praetorius J, Rutzler MR, Fenton RA. Phosphorylation of aquaporin-2 regulates its endocytosis and protein-protein interactions. Proc Natl Acad Sci USA. 2010;107:424–429. doi: 10.1073/pnas.0910683107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mundell SJ, Luo J, Benovic JL, Conley PB, Poole AW. Distinct clathrin-coated pits sort different G protein-coupled receptor cargo. Traffic. 2006;7:1420–1431. doi: 10.1111/j.1600-0854.2006.00469.x. [DOI] [PubMed] [Google Scholar]
- 55.Nagai J, Christensen EI, Morris SM, Willnow TE, Cooper JA, Nielsen R. Mutually dependent localization of megalin and Dab2 in the renal proximal tubule. Am J Physiol Renal Physiol. 2005;289:F569–F576. doi: 10.1152/ajprenal.00292.2004. [DOI] [PubMed] [Google Scholar]
- 56.Naim HY, Dodds DT, Brewer CB, Roth MG. Apical and basolateral coated pits of MDCK cells differ in their rates of maturation into coated vesicles, but not in the ability to distinguish between mutant hemagglutinin proteins with different internalization signals. J Cell Biol. 1995;129:1241–1250. doi: 10.1083/jcb.129.5.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Naslavsky N, Weigert R, Donaldson JG. Characterization of a nonclathrin endocytic pathway: membrane cargo and lipid requirements. Mol Biol Cell. 2004;15:3542–3552. doi: 10.1091/mbc.E04-02-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Naslavsky N, Weigert R, Donaldson JG. Convergence of non-clathrin- and clathrin-derived endosomes involves Arf6 inactivation and changes in phosphoinositides. Mol Biol Cell. 2003;14:417–431. doi: 10.1091/mbc.02-04-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nichols BJ, Kenworthy AK, Polishchuk RS, Lodge R, Roberts TH, Hirschberg K, Phair RD, Lippincott-Schwartz J. Rapid cycling of lipid raft markers between the cell surface and Golgi complex. J Cell Biol. 2001;153:529–541. doi: 10.1083/jcb.153.3.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Okamoto CT, Song W, Bomsel M, Mostov KE. Rapid internalization of the polymeric immunoglobulin receptor requires phosphorylated serine 726. J Biol Chem. 1994;269:15676–15682. [PubMed] [Google Scholar]
- 61.Olsen O, Liu H, Wade JB, Merot J, Welling PA. Basolateral membrane expression of the Kir 2.3 channel is coordinated by PDZ interaction with Lin-7/CASK complex. Am J Physiol Cell Physiol. 2002;282:C183–C195. doi: 10.1152/ajpcell.00249.2001. [DOI] [PubMed] [Google Scholar]
- 62.Picciano JA, Ameen N, Grant BD, Bradbury NA. Rme-1 regulates the recycling of the cystic fibrosis transmembrane conductance regulator. Am J Physiol Cell Physiol. 2003;285:C1009–C1018. doi: 10.1152/ajpcell.00140.2003. [DOI] [PubMed] [Google Scholar]
- 63.Puthenveedu MA, von Zastrow M. Cargo regulates clathrin-coated pit dynamics. Cell. 2006;127:113–124. doi: 10.1016/j.cell.2006.08.035. [DOI] [PubMed] [Google Scholar]
- 64.Qin XS, Tsukaguchi H, Shono A, Yamamoto A, Kurihara H, Doi T. Phosphorylation of nephrin triggers its internalization by raft-mediated endocytosis. J Am Soc Nephrol. 2009;20:2534–2545. doi: 10.1681/ASN.2009010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Raiborg C, Stenmark H. The ESCRT machinery in endosomal sorting of ubiquitylated membrane proteins. Nature. 2009;458:445–452. doi: 10.1038/nature07961. [DOI] [PubMed] [Google Scholar]
- 66.Rappoport JZ, Simon SM. Endocytic trafficking of activated EGFR is AP-2 dependent and occurs through preformed clathrin spots. J Cell Sci. 2009;122:1301–1305. doi: 10.1242/jcs.040030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Riquier AD, Lee DH, McDonough AA. Renal NHE3 and NaPi2 partition into distinct membrane domains. Am J Physiol Cell Physiol. 2009;296:C900–C910. doi: 10.1152/ajpcell.00526.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Roth AF, Wan J, Bailey AO, Sun B, Kuchar JA, Green WN, Phinney BS, Yates JR, 3rd, Davis NG. Global analysis of protein palmitoylation in yeast. Cell. 2006;125:1003–1013. doi: 10.1016/j.cell.2006.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rotin D, Kanelis V, Schild L. Trafficking and cell surface stability of ENaC. Am J Physiol Renal Physiol. 2001;281:F391–F399. doi: 10.1152/ajprenal.2001.281.3.F391. [DOI] [PubMed] [Google Scholar]
- 70.Sabharanjak S, Sharma P, Parton RG, Mayor S. GPI-anchored proteins are delivered to recycling endosomes via a distinct cdc42-regulated, clathrin-independent pinocytic pathway. Dev Cell. 2002;2:411–423. doi: 10.1016/s1534-5807(02)00145-4. [DOI] [PubMed] [Google Scholar]
- 71.Scheiffele P, Verkade P, Fra AM, Virta H, Simons K, Ikonen E. Caveolin-1 and -2 in the exocytic pathway of MDCK cells. J Cell Biol. 1998;140:795–806. doi: 10.1083/jcb.140.4.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schonteich E, Wilson GM, Burden J, Hopkins CR, Anderson K, Goldenring JR, Prekeris R. The Rip11/Rab11-FIP5 and kinesin II complex regulates endocytic protein recycling. J Cell Sci. 2008;121:3824–3833. doi: 10.1242/jcs.032441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schwake M, Friedrich T, Jentsch TJ. An internalization signal in ClC-5, an endosomal Cl-channel mutated in dent’s disease. J Biol Chem. 2001;276:12049–12054. doi: 10.1074/jbc.M010642200. [DOI] [PubMed] [Google Scholar]
- 74.Semerdjieva S, Shortt B, Maxwell E, Singh S, Fonarev P, Hansen J, Schiavo G, Grant BD, Smythe E. Coordinated regulation of AP2 uncoating from clathrin-coated vesicles by rab5 and hRME-6. J Cell Biol. 2008;183:499–511. doi: 10.1083/jcb.200806016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sharma M, Pampinella F, Nemes C, Benharouga M, So J, Du K, Bache KG, Papsin B, Zerangue N, Stenmark H, Lukacs GL. Misfolding diverts CFTR from recycling to degradation: quality control at early endosomes. J Cell Biol. 2004;164:923–933. doi: 10.1083/jcb.200312018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sigismund S, Argenzio E, Tosoni D, Cavallaro E, Polo S, Di Fiore PP. Clathrin-mediated internalization is essential for sustained EGFR signaling but dispensable for degradation. Dev Cell. 2008;15:209–219. doi: 10.1016/j.devcel.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 77.Sorkin A, Goh LK. Endocytosis and intracellular trafficking of ErbBs. Exp Cell Res. 2009;315:683–696. doi: 10.1016/j.yexcr.2008.07.029. [DOI] [PubMed] [Google Scholar]
- 78.Straight SW, Chen L, Karnak D, Margolis B. Interaction with mLin-7 alters the targeting of endocytosed transmembrane proteins in mammalian epithelial cells. Mol Biol Cell. 2001;12:1329–1140. doi: 10.1091/mbc.12.5.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Subramanya AR, Liu J, Ellison DH, Wade JB, Welling PA. WNK4 diverts the thiazide-sensitive NaCl cotransporter to the lysosome and stimulates AP-3 interaction. J Biol Chem. 2009;284:18471–18480. doi: 10.1074/jbc.M109.008185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tebar F, Sorkina T, Sorkin A, Ericsson M, Kirchhausen T. Eps15 is a component of clathrin-coated pits and vesicles and is located at the rim of coated pits. J Biol Chem. 1996;271:28727–28730. doi: 10.1074/jbc.271.46.28727. [DOI] [PubMed] [Google Scholar]
- 81.Tosoni D, Puri C, Confalonieri S, Salcini AE, De Camilli P, Tacchetti C, Di Fiore PP. TTP specifically regulates the internalization of the transferrin receptor. Cell. 2005;123:875–888. doi: 10.1016/j.cell.2005.10.021. [DOI] [PubMed] [Google Scholar]
- 82.Traub LM. Tickets to ride: selecting cargo for clathrin-regulated internalization. Nat Rev Mol Cell Biol. 2009;10:583–596. doi: 10.1038/nrm2751. [DOI] [PubMed] [Google Scholar]
- 83.Traub LM, Lukacs GL. Decoding ubiquitin sorting signals for clathrin-dependent endocytosis by CLASPs. J Cell Sci. 2007;120:543–553. doi: 10.1242/jcs.03385. [DOI] [PubMed] [Google Scholar]
- 84.Tzaban S, Massol RH, Yen E, Hamman W, Frank SR, Lapierre LA, Hansen SH, Goldenring JR, Blumberg RS, Lencer WI. The recycling and transcytotic pathways for IgG transport by FcRn are distinct and display an inherent polarity. J Cell Biol. 2009;185:673–684. doi: 10.1083/jcb.200809122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.van der Sluijs P, Hull M, Webster P, Male P, Goud B, Mellman I. The small GTP-binding protein rab4 controls an early sorting event on the endocytic pathway. Cell. 1992;70:729–740. doi: 10.1016/0092-8674(92)90307-x. [DOI] [PubMed] [Google Scholar]
- 86.Verkade P, Harder T, Lafont F, Simons K. Induction of caveolae in the apical plasma membrane of Madin-Darby canine kidney cells. J Cell Biol. 2000;148:727–739. doi: 10.1083/jcb.148.4.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vilhardt F, Nielsen M, Sandvig K, van Deurs B. Urokinase-type plasminogen activator receptor is internalized by different mechanisms in polarized and nonpolarized Madin-Darby canine kidney epithelial cells. Mol Biol Cell. 1999;10:179–195. doi: 10.1091/mbc.10.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang E, Brown PS, Aroeti B, Chapin SJ, Mostov KE, Dunn KW. Apical and basolateral endocytic pathways of MDCK cells meet in acidic common endosomes distinct from a nearly-neutral apical recycling endosome. Traffic. 2000;1:480–493. doi: 10.1034/j.1600-0854.2000.010606.x. [DOI] [PubMed] [Google Scholar]
- 89.Wang H, Traub LM, Weixel KM, Hawryluk MJ, Shah N, Edinger RS, Perry CJ, Kester L, Butterworth MB, Peters KW, Kleyman TR, Frizzell RA, Johnson JP. Clathrin-mediated endocytosis of the epithelial sodium channel. Role of epsin. J Biol Chem. 2006;281:14129–14135. doi: 10.1074/jbc.M512511200. [DOI] [PubMed] [Google Scholar]
- 90.Warren RA, Green FA, Stenberg PE, Enns CA. Distinct saturable pathways for the endocytosis of different tyrosine motifs. J Biol Chem. 1998;273:17056–17063. doi: 10.1074/jbc.273.27.17056. [DOI] [PubMed] [Google Scholar]
- 91.Weinman EJ, Cunningham R, Wade JB, Shenolikar S. The role of NHERF-1 in the regulation of renal proximal tubule sodium-hydrogen exchanger 3 and sodium-dependent phosphate cotransporter 2a. J Physiol. 2005;567:27–32. doi: 10.1113/jphysiol.2005.086777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wiemuth D, Ke Y, Rohlfs M, McDonald FJ. Epithelial sodium channel (ENaC) is multiubiquitinated at the cell surface. Biochem J. 2007;405:147–155. doi: 10.1042/BJ20060747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wilde A, Beattie EC, Lem L, Riethof DA, Liu SH, Mobley WC, Soriano P, Brodsky FM. EGF receptor signaling stimulates SRC kinase phosphorylation of clathrin, influencing clathrin redistribution and EGF uptake. Cell. 1999;96:677–687. doi: 10.1016/s0092-8674(00)80578-4. [DOI] [PubMed] [Google Scholar]
- 94.Wiley HS. Anomalous binding of epidermal growth factor to A431 cells is due to the effect of high receptor densities and a saturable endocytic system. J Cell Biol. 1988;107:801–810. doi: 10.1083/jcb.107.2.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wilson FH, Disse-Nicodeme S, Choate KA, Ishikawa K, Nelson-Williams C, Desitter I, Gunel M, Milford DV, Lipkin GW, Achard JM, Feely MP, Dussol B, Berland Y, Unwin RJ, Mayan H, Simon DB, Farfel Z, Jeunemaitre X, Lifton RP. Human hypertension caused by mutations in WNK kinases. Science. 2001;293:1107–1112. doi: 10.1126/science.1062844. [DOI] [PubMed] [Google Scholar]
- 96.Wolfe BL, Trejo J. Clathrin-dependent mechanisms of G protein-coupled receptor endocytosis. Traffic. 2007;8:462–470. doi: 10.1111/j.1600-0854.2007.00551.x. [DOI] [PubMed] [Google Scholar]
- 97.Yang LE, Maunsbach AB, Leong PK, McDonough AA. Differential traffic of proximal tubule Na+ transporters during hypertension or PTH: NHE3 to base of microvilli vs. NaPi2 to endosomes. Am J Physiol Renal Physiol. 2004;287:F896–F906. doi: 10.1152/ajprenal.00160.2004. [DOI] [PubMed] [Google Scholar]
- 98.Yang LE, Maunsbach AB, Leong PK, McDonough AA. Redistribution of myosin VI from top to base of proximal tubule microvilli during acute hypertension. J Am Soc Nephrol. 2005;16:2890–2896. doi: 10.1681/ASN.2005040366. [DOI] [PubMed] [Google Scholar]
- 99.Yudowski GA, Puthenveedu MA, Henry AG, von Zastrow M. Cargo-mediated regulation of a rapid Rab4-dependent recycling pathway. Mol Biol Cell. 2009;20:2774–2784. doi: 10.1091/mbc.E08-08-0892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zagorska A, Pozo-Guisado E, Boudeau J, Vitari AC, Rafiqi FH, Thastrup J, Deak M, Campbell DG, Morrice NA, Prescott AR, Alessi DR. Regulation of activity and localization of the WNK1 protein kinase by hyperosmotic stress. J Cell Biol. 2007;176:89–100. doi: 10.1083/jcb.200605093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhou B, Zhuang J, Gu D, Wang H, Cebotaru L, Guggino WB, Cai H. WNK4 enhances the degradation of NCC through a sortilin-mediated lysosomal pathway. J Am Soc Nephrol. 2010;21:82–92. doi: 10.1681/ASN.2008121275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhou R, Patel SV, Snyder PM. Nedd4–2 catalyzes ubiquitination and degradation of cell surface ENaC. J Biol Chem. 2007;282:20207–20212. doi: 10.1074/jbc.M611329200. [DOI] [PubMed] [Google Scholar]