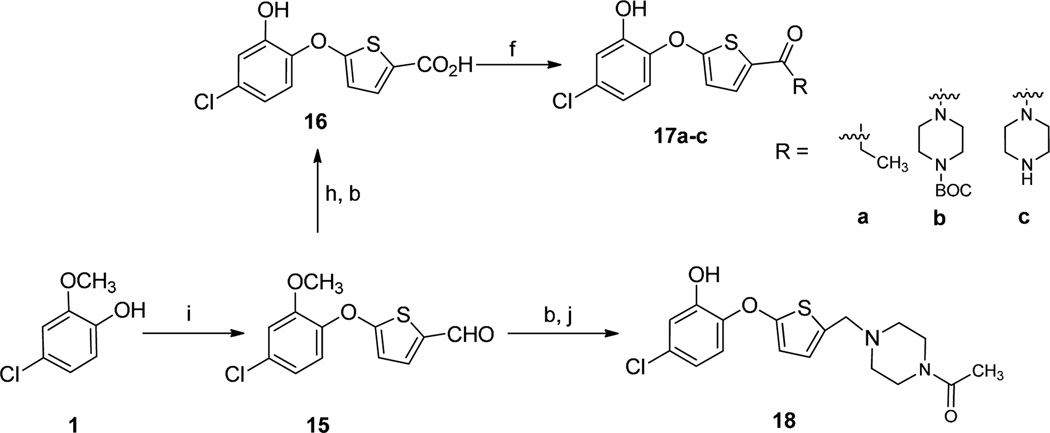
Scheme 3.
Synthesis of thienyl phenyl ethers. Reagents and conditions: (b) BBr3, CH2Cl2, −78 °C to room temp, 6 h; (f) amine, HOBt, EDCI, DIEA, CH2Cl2, 0 °C to rt, overnight, 40–60%; (h) NaClO2, DMSO, NaH2PO4, THF/t-BuOH/H2O; (i) 5- bromothiophene-2-carbaldehyde, K2CO3, DMSO, 80 °C, overnight, 60%; (j) amine, NaBH(OAc)3, AcOH, CH2Cl2, 0 °C to room temperature, 1 h.