Abstract
3′,5′-cyclic adenosine monophosphate (cAMP) dependent protein kinase or protein kinase A (PKA) has served as a prototype for the large family of protein kinases that are crucially important for signal transduction in eukaryotic cells. The PKA catalytic subunits Cα and Cβ, encoded by the two genes PRKACA and PRKACB, respectively, are among the best understood and characterized human kinases. Here we have studied the evolution of this gene family in chordates, arthropods, mollusks and other animals employing probabilistic methods and show that Cα and Cβ arose by duplication of an ancestral PKA catalytic subunit in a common ancestor of vertebrates. The two genes have subsequently been duplicated in teleost fishes. The evolution of the PRKACG retroposon in simians was also investigated. Although the degree of sequence conservation in the PKA Cα/Cβ kinase family is exceptionally high, a small set of signature residues defining Cα and Cβ subfamilies were identified. These conserved residues might be important for functions that are unique to the Cα or Cβ clades. This study also provides a good example of a seemingly simple phylogenetic problem which, due to a very high degree of sequence conservation and corresponding weak phylogenetic signals, combined with problematic nonphylogenetic signals, is nontrivial for state-of-the-art probabilistic phylogenetic methods.
Introduction
Protein kinases are enzymes that catalyze the transfer of a phosphate group from adenosine 5′-triphosphate (ATP) to a serine, threonine, tyrosine or other residue on a substrate. Most eukaryotic protein kinases derive from a common ancestor kinase, and share the same core catalytic domain [1].
PKA (EC 2.7.11.11) is a serine/threonine kinase which is ubiquitously expressed in the human body. It is involved in many intracellular signaling events, and its function, specificity, and downstream effects depend on factors such as subcellular localization, expression of a number of isoforms and physio-chemical features [2], [3]. The inactive form of PKA is a heterotetrameric holoenzyme consisting of a regulatory (R) subunit dimer binding to two catalytic (C) subunits [4]. During activation, cAMP binds cooperatively to two sites termed A and B on each R subunit. In the inactive holoenzyme, only the B site is exposed and available for cAMP binding. When occupied, this enhances the binding of cAMP to the A site which leads to an intramolecular conformational change and the release of the R subunit dimer. The two C monomers are then free to phosphorylate relevant C substrates in the cytosol and nucleus [5]–[7]. Thus, a major function of the R subunit is to inhibit the phosphotransferase activity of the C subunits through direct interactions.
Several variants of the C and R subunits have been identified in human cells. Four R subunits designated RIα, RIβ, RIIα, and RIIβ are transcribed from separate genes [8]. PKA holoenzymes containing RI and RII subunits are designated PKA type I and II, respectively [9], [10]. Protein-protein interactions and organization of signal transduction pathways is required to obtain specificity in space and time. Protein kinases are localized to the relevant subcellular sites through anchoring, scaffolding and adapter protein activity. Major organizers of the cAMP signaling pathway are the A kinase anchoring proteins (AKAPs), which both function as scaffolding proteins and attach PKA to subcellular structures [11], [12]. Initially, AKAPs were shown to interact with the RII subunits [13]. Later, dual specific AKAPs binding both RII and RI, as well as AKAPs binding only RI, have been identified, demonstrating that both PKA type I and II may be tethered to subcellular compartments in the cell [14]–[16].
Five different human C subunit genes have been identified; PRKACA, PRKACB, PRKACG, PRKX, and PRKY [2], [17]–[19], all within the AGC group of kinases, which contains the cyclic-nucleotide-dependent family (PKA and PKG), the protein kinase C family, the β-adrenergic receptor kinase, the ribosomal S6 family and some other relatives of these kinases [1], [20]. Three of the human C subunit genes, PRKACA, PRKACB, and PRKX, have been demonstrated to be transcribed and translated into functional protein kinases, termed PKA Cα, PKA Cβ, and PRKX, respectively. Cα exhibits two splice variants, Cα1 [21] and Cα2 [22]–[24], by employing two alternative 5′ exons in PRKACA (Fig. 1A). Whereas Cα1 is ubiquitously expressed in man, Cα2 is exclusively expressed in the sperm cell and has been shown to be essential for sperm motility and fertilization [24]–[27]. A number of isoforms of human PRKACB have been identified with alternative splicing of exons 5′ of exon 2 (Fig. 1A), encoding at least the following proteins: Cβ1, Cβ2, Cβ3, Cβ4, Cβ3ab, Cβ3b, Cβ3abc, Cβ4ab, Cβ4b, and Cβ4abc [28]–[33]. In addition, Cβ variants formed by skipping of exon 4 are expressed in the brain of higher primates. These C subunits bind the R subunit in a cAMP-independent fashion [34].
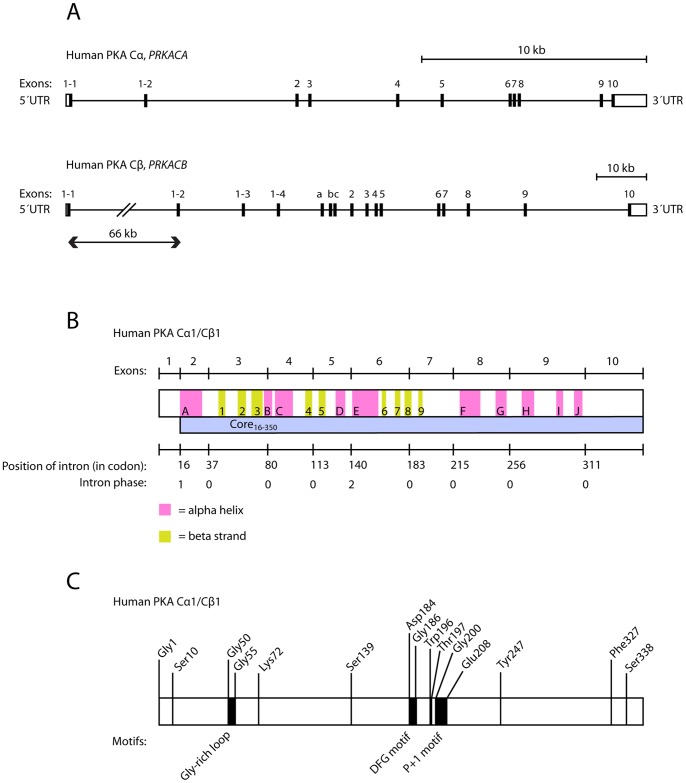
Figure 1. Gene structure and overview of the human PKA catalytic subunits Cα and Cβ encoded by the PRKACA and PRKACB genes, respectively.
A Exons, introns and 3′ and 5′ untranslated regions (UTRs) of the PKA catalytic subunit genes are shown. The human Cα gene (PRKACA) is located at chromosome 19p13.1 (reverse strand) and has a length of approximately 26 000 nucleotides (nt). Alternative transcription start sites give rise to two splice variants known as Cα1 and Cα2 (formerly known as CαS). Both splice variants comprise exons 2 to 10 and in addition a 5′ exon 1–1 or 1–2 in Cα1 and Cα2, respectively. The human Cβ gene (PRKACB) is located at chromosome 1p31.1 (forward strand), with a length of approximately 160 000 nt. Alternative splicing of exons 1–1, 1–2, 1–3 and 1–4 give rise to the splice variants Cβ1, Cβ2, Cβ3 and Cβ4, respectively. In addition, three short exons, a, b and c, have been shown to be included in the transcript in various combinations. All known enzymatically active isoforms of Cβ comprise exons 2 to 10. B Human PKA Cα1 consists of ten α-helix and nine β-strand secondary structure elements [76]. The figure (middle box) gives the location of α-helices (pink, A–J) and β-strands (yellow, 1–9) relative to the ten exons and 351 encoded codons of the Cα1 isoform. The locations of the boundaries between exons are given on the upper line. The codons corresponding to the nine introns, as well as their intron phases, are given on the lower line. The intron phase is defined as the position of the intron within a codon, with phase 0, 1, or 2 lying before the first base, after the first base, or after the second base, respectively. Human PKA Cβ1 has the same length as Cα1, and the two proteins are differing at only 25 amino acid positions (92.9% sequence identity), strongly suggesting that the overall 3D structures, including secondary structure elements, are close to identical. The position and intron phases for the nine introns are also conserved between human Cα1 and Cβ1. The sequence segment corresponding to exons 2–10, termed Core16–350, is shown as a blue bar. C The function of selected important residues and motifs in human PKA catalytic subunits has previously been elucidated in the literature. All listed residues, and their numbering, are identical in Cα1 and Cβ1, but the research describing these residues and motifs has mainly been performed on Cα. Numbering of amino acids is given for mature Cα1 and Cβ1 (with N-terminal Met removed), both encoding 350 residues. Gly1 is found to be posttranslationally modified by myristoylation [77]. Ser10, Ser139 and Ser338 are well characterized phosphorylation sites [78], [79]. The Gly-rich loop (Gly50–Gly55) plays an important role in phosphoryl transfer [80]–[82]. Lys72 and Asp184 are crucial for ATP and Mg2+ binding in the active site [81] and the DFG motif (Asp184–Gly186) is conserved in most kinases. The conformation of the motif is critical for the functional state of the kinase [83], [84]. Phe327 is the only residue outside of the kinase core binding to the adenine of ATP [82]. Trp196 is an essential residue for R subunit binding and phosphorylation of Thr197 is necessary for the enzyme to assume the active conformation, thereby facilitating catalysis as well as R subunit binding [5], [85]. The hydrophobic P+1 motif (Gly200–Glu208) is important for the structure of the enzyme, as well as for substrate recognition [80], [86]. Tyr247 competes with cAMP for R subunit binding [5].
PRKX, which is a protein kinase encoded from the X-chromosome, also binds RIα in a cAMP sensitive fashion [19]. PRKX and PRKY are 94% identical and have unknown functions [18], [20]. PRKACG is a retroposon lacking introns and may be a pseudogene [35]. Whereas mRNA from PRKACG is solely, but ubiquitously, transcribed in the testis [17], the corresponding protein Cγ has never been identified. In vitro experiments on expressed Cγ have revealed a functional kinase with variant-specific properties. The Cγ protein kinase is, in contrast to Cα, and most probably Cβ, not inhibited by the Protein Kinase Inhibitor (PKI), and it requires higher concentrations of cAMP for dissociation from PKA type I holoenzymes [36], [37]. The in vivo function of PKA Cγ, if the protein exists, remains to be elucidated.
The PRKACA and PRKACB genes share identical positions and intron phases for all nine introns, and are very likely the result of a gene duplication event (Fig. 1B). Human PKA Cα1 and Cβ1 have the same length (350 residues) and share 93% sequence identity. Many studies have elucidated the importance and function of a number of residues and short sequence segments in these kinases, and some of these are briefly summarized in Fig. 1C. The sequence identity between human PKA Cα vs. Cγ and PRKX is, 82% and 54%, respectively, and between Cα/Cβ and other human kinases below 50%. PKA C kinases in invertebrate metazoa have sequence identity with human Cα/Cβ above 77% (see below), confirming that the PKA Cα/Cβ-like kinases, not including PRKX, builds a unique and compact clade/family of kinases within the AGC-group.
A previous study explored the evolution of the R subunits of PKA [38]. Based on a multiple sequence alignment (MSA) of the most conserved region in the R subunit sequences, the phosphate-binding cassette, the authors proposed a new classification of the R subunits in place of a classification based on physiochemical properties. They also identified a signature sequence that characterizes the R subunit genes, and identified type- and subtype-specific residues. The same focused analysis of the C subunits of PKA is to our knowledge lacking. In order to get a comprehensive overview of all the known PKA genes and obtain insight into the essential residues of these proteins, we have collected, compared and performed an extensive analysis of PKA C subunit sequences from a large number of bilaterian animal species. The homologous genes PRKACA and PRKACB constitute a unique clade of kinases and their protein products are the main sources of PKA activity in the cell. Therefore, we focused our analysis on the Cα/Cβ-like kinases, including Cγ, but not the more remotely related kinases PRKX and PRKY. The main focus of this study has been on elucidating the phylogeny of vertebrate and other chordate PKA Cα/Cβ homologs, while orthologous sequences from mollusks and arthropods were included mainly to serve as outgroups in the phylogenetic analysis. We show that an ancestor C subunit was duplicated around the time of the evolution of the first vertebrate species, giving rise to the paralogous genes encoding Cα and Cβ. Further analysis on the Cα/Cβ homologs revealed signature sequences characteristic of the two paralogs. Comparison of Cα and Cβ sequences gave insight into molecular differences and possible mechanisms that may determine functional differences between these two prototype protein kinases.
Materials and Methods
Sequences of PKA Cα/Cβ Homologs
Homologous sequences of human PKA Cα/Cβ were obtained from the UniProt [39] and NCBI [40] database resources and from the Ensembl project [41] as described in Materials and Methods S1. Standard BLAST sequence searching algorithms were employed [42] and only sequences with sequence identity (protein level) compared with human PKA Cα or Cβ above 75% were included in the dataset for further analysis. The final dataset comprised 41 sequences from placental mammals, including 18 from primates, 5 marsupial sequences, 35 sequences from non-mammalian vertebrates, and 15 from invertebrates (See Materials and Methods S1 and Table S1). Both nucleotide and protein sequences, one single splice variant for each gene, were stored in FASTA format for the total 96 sequences. 81 sequences appear to be full-length, comprising exons 2 to 10 and in addition a 5′ exon chosen to correspond to human Cα1/Cβ1 when possible. Of the incomplete sequences, 8 were missing only the 5′ exon. In addition, one Petromyzon marinus sequence was missing exons 1, 7, 9, and 10, and the two sequences from Macropus eugenii were missing exons 1 and 9, and part of the 3′ end, respectively. Finally, the four cartilaginous fish sequences only contain fragments of the full-length sequence.
The sequences were aligned with MUSCLE [43] and the MSAs were viewed and edited with Jalview [44] (See Figure S1). All analysis was subsequently based on MSAs corresponding to exons 2–10 of human PKA Cα1/Cβ1 (i.e. residues 16–350, Fig. 1B) unless otherwise stated, and columns of the MSAs containing gaps were deleted. PHYLIP and NEXUS format files were generated from the FASTA files with a dedicated Perl script. The best model for nucleotide evolution was determined with ModelTest 3.7 [45] in combination with PAUP* (D. L. Swofford, 2003. PAUP*, Sinauer Associates, Sunderland, MA) as chosen by the Akaike Information Criterion (AIC). The evolutionary model selected was the general time reversal (GTR) model with a discontinuous gamma distribution (Γ) for modeling rate heterogeneity over sites and a proportion of invariant sites (I), i.e. GTR+Γ+I with four rate categories. The best fitting sequence substitution model for the protein data was determined with ProtTest 2.4 [46], and was found to be, according to the AIC, LG+Γ+I [47]. Also the second best model, JTT+Γ+I [48], was tested for phylogenetic tree construction.
Phylogenetic Analysis
Bayesian inference of phylogeny was carried out with MrBayes 3.1.2 [49], [50] with default heating parameters (three heated Markov chain Monte Carlo chains and one cold) and priors. Two simultaneous and independent runs were carried out with sampling every 10 of 500 k generations until average standard deviation of split frequencies were below 0.01. Branch lengths and majority rule consensus tree topologies were calculated after discarding a burn-in of 100 k generations after which stationarity had been reached. For all calculations presented, the final potential scale reduction factor (PSRF) was below 1.004 for all parameters.
Phylogenetic trees were also generated with the Maximum Likelihood (ML) method employing PhyML 3.0 [51] with default parameters. The robustness of each clade was estimated by a nonparametric bootstrap analysis with 1000 replicates. Gamma distribution parameters, the proportion of invariable sites, branch lengths and GTR model parameters were all optimized by the ML algorithm from the data. ML phylogenetic trees were also inferred using RAxML 7.2.6 [52], but due to the similarities of the resulting trees only the PhyML results are shown here.
Dendroscope 2.7.4 [53] was used for visualization of phylogenetic trees. Signature sequence logos were generated by applying WebLogo [54]. Calculations were carried out on the University of Oslo Titan computer cluster, mainly through the freely available Bioportal (www.bioportal.uio.no). Protein structure illustrations were generated with PyMOL (W. L. DeLano, The PyMOL Molecular Graphics System, Version 1.3, Schrödinger, LLC). Ratios of nonsynonymous and synonymous substitution rates were calculated with the KaKs_Calculator [55].
Results and Discussion
The PKA Cα/Cβ Family is Highly Conserved in Chordates
Vertebrate homologs of human PKA Cα and Cβ were extracted from public databases, including Ensembl, UniProt, and database resources provided by the NCBI. New gene models were generated for several of the homologs by careful manual curation (See details in Materials and Methods S1). Homologs, in most cases full-length sequences, were found from 21 placental mammals, the marsupial species opossum (Monodelphis domestica) and wallaby (Macropus eugenii), chicken, zebra finch (Taeniopygia guttata), the Carolina anole lizard (Anolis carolinensis), and two and six species of frogs and bony fishes, respectively. In addition, full-length sequences were obtained for the non-vertebrate chordates amphioxus (Branchiostoma floridae) and the tunicates Ciona intestinalis and Ciona savignyi, the echinoderm sea urchin (Strongylocentrotus purpuratus), the two mollusks great pond snail (Lymnaea stagnalis) and California sea hare (Aplysia californica), the hemichordate Saccoglossus kowalevskii, the nematode Caenorhabditis elegans, the sponge Amphimedon queenslandica, as well as six arthropods including honey bee, fruit fly, a tick, and a crustacean. Partial sequences were obtained for orthologs from dogfish shark (Squalus acanthias), little skate (Leucoraja erinacea) and sea lamprey (Petromyzon marinus). In all cases both the nucleotide sequences and the corresponding protein sequences were stored. All sequences are listed in Materials and Methods S1.
We were unable to detect more than a single, reasonably close, PKA Cα/Cβ homolog in any of the invertebrate species that were examined. These includes the cephalochordate amphioxus, the urochordates C. intestinalis, C. savignyi and Oikopleura dioica, the echinoderm sea urchin, as well as arthropods, mollusks, a nematode and a sponge. Two homologs were found in most vertebrates, including sea lamprey, and the chondrichthyes S. acanthias and L. erinacea, while four homologs were detected in a number of bony fishes.
The main isoforms of human PRKACA and PRKACB comprises exons 2–10 and in addition one or more 5′ exons (Fig. 1A). The length of all exons 2–10 and the positions and intron phases of all nine introns are identical in the two human genes (Fig. 1B), clearly demonstrating that these genes arose due to a gene duplication. This structure, reflecting an extreme degree of conservation, is also conserved in all chordate homologs, including the invertebrate cephalochordate amphioxus and the urochordate tunicates and in addition in the echinoderm sea urchin, the hemichordate S. kowalevskii, and the crustacean Daphnia pulex: the number of exons, their lengths and the codon phases of all introns are identical. The exceptions, apart from the intronless homologs described below, includes the medaka gene encoding a protein with Ensembl identifier ENSORLP00000018527. This gene appears to have acquired a new 88-nucleotide intron (intron phase 0) in the middle of exon 9. In addition, the basal metazoan A. queenslandica has an additional intron of 334 nucleotides that splits the coding sequence corresponding to vertebrate exon 6.
The number of codons encoded by exons 2–10 in the PRKACA/B family of genes - corresponding to residues 16–350 in human proteins PKA Cα1/Cβ1 - is consequently also identical in all species listed above. We term this sequence segment Core16–350 (Fig. 1B) in order to distinguish it from the variable 5′ exons. The genomic data for the mollusks are not yet available in the public domain and the intron/exon structure is currently unknown, but in insects the protein coding segment of the PRKACA/B homologs are contained in a single exon. Nevertheless, the insect homologs also have the same number of residues for the segment corresponding to Core16–350. An MSA of all sequences with full-length Core16–350 is shown in Figure S1. Consequently, we find that nearly all our PKA Cα/Cβ homologs from Bilateria have the same length for the Core16–350, and with variable length N-termini corresponding to the multitude of isoforms. The only exceptions are a single-residue insertion after human PKA Cα1 Lys63, in the middle of exon 3, in both homologs from mollusks and a single-residue insertion after human PKA Cα1 Ala38 in a fast-evolving sequence from opossum (identifier ENSMODP00000015141). These insertions are in loop structures in PKA Cα1 (See e.g. [56] for the 3D structure) and are expected to be compatible with an unchanged overall 3D structure of the kinase.
Pairwise sequence identity between any of the bilaterian PKA Cα/Cβ homologs described above is always above 77% for the 335 residues of the Core16–350 at the amino acid level. Leaving out the fast-evolving sequences from marsupials and a single fast-evolving zebrafish sequence (Q7T374), the sequence identities are always above 80% and 87% within the chordates and vertebrates, respectively. This demonstrates very strong purifying selection and an exceptionally high degree of sequence conservation for this protein family.
The PKA Cα/Cβ Gene Family Contains Several Putative Retroposons
In addition to the PRKACA/B homologs that have conserved exon/intron structure in chordates and several other deuterostomes, and the arthropod homologs, also with several exons, but with the protein coding segment contained in a single exon, a number of intronless PRKACA/B homologs are found in vertebrate genomes. Among these are human PRKACG, located on chromosome 9 between the genes PIP5K1B and FXN, but transcribed in the opposite direction. Intronless PRKACG has the same number of codons as the PKA Cα1-like transcript of PRKACA and is conserved in the great apes, in chimpanzee, gorilla, and orangutan and in the Old World monkeys rhesus macaque and hamadryas baboon (Sequences are listed in Materials and Methods S1). Genome browsing at the Ensembl resource also shows the synteny to be conserved in these species with PIP5K1B and FXN being transcribed in one direction and PRKACG, located between these two genes, in the opposite. Between PIP5K1B and FXN in the gibbon (Nomascus leucogenys) genome, a species more closely related to great apes than the Old World monkeys, there is no full-length PRKACG ortholog, but instead a putative PRKACA/B pseudogene with several frame shifting mutations. We find no evidence of PRKACG orthologs in prosimian primates such as greater galago (Otolemur garnettii), tarsier (Tarsius syrichta), or gray mouse lemur (Microcebus murinus), although the last two of these have genomes that are still fragmented and with undisclosed synteny around the genes PIP5K1B and FXN. In the common marmoset (Callithrix jacchus) genome, there is a fragment, most likely not protein-coding, of PRKACG between PIP5K1B and FXN, corresponding to PKA Cα1 residues 21–350. Finally, in the mouse and dog genomes, PIP5K1B and FXN are neighboring genes being transcribed in the same direction, but without any sign of a PRKACA/B homolog in this region.
These findings strongly support the previous suggestion that PRKACG is a retroposon due to a PKA Cα1-type transcript [35] that has been inserted between PIP5K1B and FXN in a common ancestor of great apes and Old and New World monkeys. The putative PRKACG transcripts could potentially give rise to functional kinases in all great apes and in the Old World monkeys, but not in gibbons and the New World monkey marmoset where there are mutations disrupting the reading frame in the PRKACG retroposon. In the marmoset genome, there is in addition to the putative PRKACG pseudogene on chromosome 1, a second retroposon (Ensembl identifier ENSCJAP00000040924) related to PKA Cα1 on chromosome 2. This gene was not found in other primates.
In addition to the PKA Cα1-like retroposons in primates, we found intronless PRKACA homologs in the two sequenced genomes of marsupials, the wallaby kangaroo (M. eugenii) and the Brazilian opossum (M. domestica) (See Materials and Methods S1). Also these putative retroposons appear to be derived from a PKA Cα1-type transcript, but are otherwise unrelated to primate PRKACG.
Elucidating the Phylogeny of the PKA Cα/Cβ Family is Nontrivial Due to High Sequence Conservation
The distribution of PKA Cα/Cβ homologs in Bilateria, as well as phylogenetic trees generated with unsophisticated hierarchical clustering methods (results not shown), suggest that this gene family has expanded through repeated gene duplication events in vertebrates. However, despite numerous attempts, state-of-the-art probabilistic methods, both Bayesian inference and maximum likelihood (ML) methods, were not able to generate a statistically strongly supported phylogenetic tree for the full PKA Cα/Cβ family from the complete data set. After careful analysis of the data (vide infra), we were nevertheless able to derive reliable phylogenies for this gene family by dividing the data into subsets. The final phylogenetic trees were generated as follows: an MSA was generated from the nucleotide sequences corresponding to the Core16–350 segment for selected vertebrates, amphioxus, sea urchin and fruit fly. After removal of all nucleotides at codon position 3, the dataset was employed to generate Bayesian inference and ML trees, with identical topology, for the PKA Cα/Cβ homologs with good Bayesian posterior probabilities and bootstrap support for the major nodes. The phylogram was rooted with the sea urchin and fruit fly as outgroups (Fig. 2). Bayesian inference methods were also used to generate a phylogenetic tree of 22 vertebrate PKA Cα orthologs employing human and mouse PKA Cβ as outgroups (Fig. 3A). Similarly, a tree with 26 PKA Cβ orthologs was generated with human and mouse PKA Cα as outgroups (Fig. 3B). These trees, and trees from the corresponding ML analysis, are based on MSAs for the nucleotide sequences with all three codon positions included.
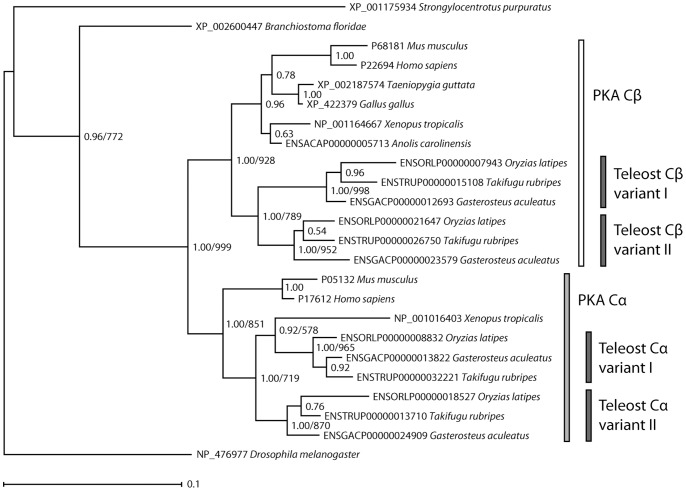
Figure 2. Phylogenetic relationships among the PKA catalytic subunit homologs in chordates.
The Cα and Cβ paralogs are a result of a gene duplication in a common ancestor of vertebrates. Subsequent duplications of Cα and Cβ in a teleost fish ancestor have resulted in four PKA catalytic subunits in these organisms. The Bayesian inference tree is based on the nucleotide sequences (codon positions 1 and 2 only, GTR+Γ+I model) of exons 2 to 10 which corresponds to a multiple sequence alignment with no gaps. The phylogram is shown with estimated branch lengths proportional to the number of substitutions at each site, as indicated by the scale bar. The arthropod fruit fly (D. melanogaster) and the echinoderm sea urchin (S. purpuratus) have been set as outgroups. Bayesian posterior probabilities are shown for each node. The topology of a maximum likelihood (ML) tree generated with the same data set and model was identical to the Bayesian inference tree. ML bootstrap values are shown for selected nodes (1000 replications). The sequences of human and mouse PKA Cα and Cβ and the homologs from amphioxus (B. floridae), zebra finch (T. guttata), chicken (G. gallus), the frog X. tropicalis, the lizard A. carolinensis, medaka (O. latipes), the pufferfish T. rubripes, and stickleback (G. aculeatus) are described in Materials and Methods S1. The X. tropicalis Cα and A. carolinensis Cβ are incorrectly placed (See discussion and Fig. 3).
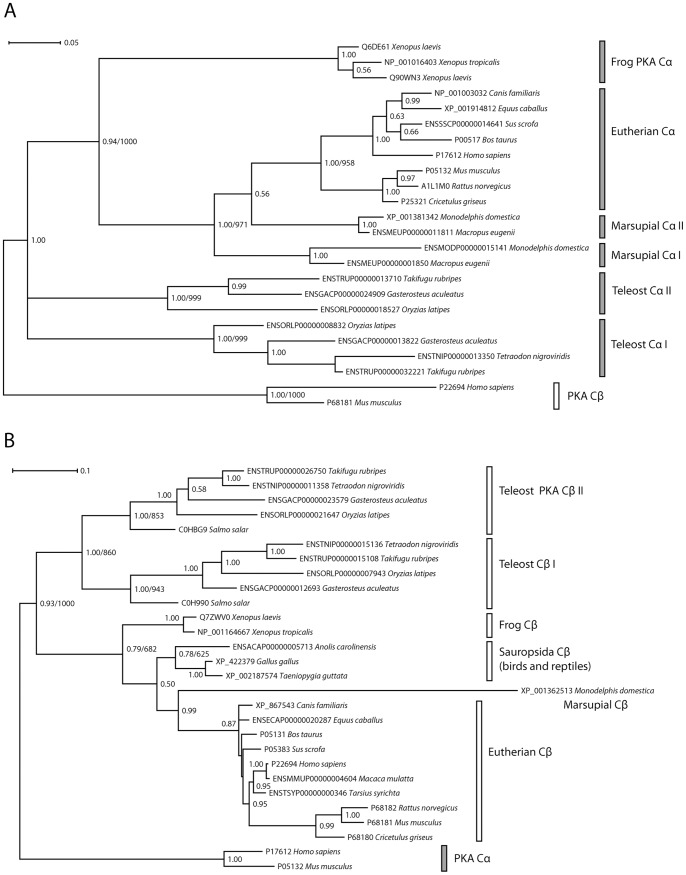
Figure 3. The Bayesian inference trees for vertebrate PKA Cα and Cβ both closely reflects the evolutionary relationships among these organisms.
A Phylogenetic analysis of Cα orthologs resulted in a tree that was rooted with human and mouse Cβ as outgroups. The tree was based on the nucleotide sequences of exons 2 to 8 (all codon positions, GTR+Γ+I model). B Phylogenetic analysis of Cβ orthologs was performed employing nucleotide sequence data (all codon positions, exons 2 to 10, GTR+Γ+I model). The resulting tree was rooted with human and mouse Cα as outgroups. In both trees, branch lengths are shown as substitutions per site, with scale indicated by the scale bars. Bayesian posterior probabilities are given for each node and ML bootstrap values (1000 replications) are shown for selected nodes where the clades are identical in the Bayesian and ML analysis. In addition to organisms found in Fig. 2, representative sequences from the following species were included: eutherian mammals rhesus macaque (M. mulatta), tarsier (T. syrichta), dog (C. familiaris), horse (E. caballus), pig (S. scrofa), cow (B. taurus), rat (R. norvegicus), and hamster (C. griseus), marsupial mammals wallaby (M. eugenii) and opossum (M. domestica), the frog X. laevis, the pufferfish T. nigroviridis and Atlantic salmon (S. salar). See Materials and Methods S1 for the sequence data.
Neither Bayesian inference nor ML methods, generally accepted to be the most accurate [57], [58], were able to generate a reliable phylogeny for the PKA Cα/Cβ family from the full data set. This was not due to unreliable MSAs as only three of the sequences in the original data set each had single codon insertions (vide supra) and manual removal of these was trivial. The problem, however, appears to be a combination of weak phylogenetic signals and problematic nonphylogenetic signals in the data. Due to the very high level of sequence conservation, the protein data set contains few phylogenetically informative sites, i.e. amino acid sites that favor one phylogenetic tree topology over others. As an example, for the 22 chordate taxa used to generate the tree in Fig. 2, the corresponding amino acid data set has 335 columns/sites. Of these, only 58 sites are phylogenetically informative, while 255 are fully conserved and identical for all taxa and the remaining 22 are autapomorphic. Unsurprisingly, protein data phylogenetic trees generated with two recommended substitution models (LG/JTT+Γ+I) and well-tested ML programs (PhyML/RAxML) were overall fairly similar, but with very poor bootstrap support. The trees generated with various subsets of the available taxa were incongruent and also in several cases inconsistent with the known evolutionary relationship between the various chordate species.
In order to secure a stronger phylogenetic signal [59], the nucleotide data set was employed for deriving the phylogenetic relations. For the 22 chordate taxa in Fig. 2, there are 77, 33, and 312 phylogenetically informative sites (out of 335 sites in total) for codon position 1, 2, and 3, respectively. The low number of informative sites at codon position 1 and 2 reflects the high degree of conservation at the amino acid level, while the high number at codon position 3 (93%) reflects the large time-span since the common ancestor of these genes. For all tested nucleotide data sets, the GTR+Γ+I model was predicted to be superior. While using the nucleotide sequences for deriving the phylogenetic relationships ensures a stronger phylogenetic signal, in particular the data from codon position 3, due to the degeneracy of the genetic code, may be severely mutationally saturated due to reversions and convergences that erase the true phylogenetic signal. This will especially be problematic for inferring ancient phylogenies [59], where long branch attraction (LBA), i.e. a tendency for grouping of lineages with long branches irrespective of their true relationships, may lead to misleading phylogenies [60]. In particular, fast-evolving genes may artificially occur too deeply in the tree due to LBA towards the outgroups.
As for the protein data set, trees generated with subsets of the available taxa were highly incongruent and in several cases with pronounced LBA, clearly demonstrating a strong nonphylogenetic signal. In order to secure minimal nonphylogenetic signals and reduce LBA, codon position 3 sites were removed from the data set used to generate the phylogeny in Fig. 2. In addition, fast-evolving taxa such as the tunicate [61], marsupial PKA Cα/Cβ homologs and the primate PKA Cγ homologs were left out of the data set. Fig. 2 is expected to describe the ancient evolution of the PKA Cα/Cβ family in chordates correctly, with the gene duplications in the common ancestor of vertebrates as well as in teleost fishes supported by high Bayesian posterior probabilities and strong bootstrap support for the ML analysis.
In order to better describe the evolution of the two PKA Cα and PKA Cβ paralogs in vertebrates separately, the trees in Fig. 3 were generated. Reliable phylogenies could not be generated from a data set containing codon positions 1 and 2 only, as in Fig. 2, most likely due to weak phylogenetic signals in the data. However, for the relatively recent phylogenetic relations in Fig. 3, the saturation at codon position 3 is expected to be less severe and all three codon positions were included in the data set for analysis. In Fig. 2, there are two errors due to nonphylogenetic signal. These are the placement of frog PKA Cα in a clade together with teleost fish and the erroneous lizard/frog PKA Cβ clade. Both these errors are corrected in Fig. 3 upon inclusion of codon position 3 data. The most ancient splittings, however, are unreliable in Fig. 3, in particular the description of the branching between the tetrapod and teleost fish PKA Cα homologs as a multifurcation (Bayesian analysis) or two bipartitions with extremely poor bootstrap support (ML analysis, not shown) in Fig. 3A.
In conclusion, while the phylogenies of the PKA Cα and PKA Cβ subfamilies, especially within the tetrapods, appears to be correctly inferred from the full nucleotide dataset (Fig. 3), the ancient evolution of the PKA Cα/Cβ family in chordates is correctly described with cladistic methods only after removal of codon position 3 data (Fig. 2). The protein data set contains limited phylogenetic information and does not give reliable phylogenies in chordates. No analysis based on partitioned data, for example according to codon position, was attempted, as this is likely to lead to overparametrization.
Phylogenetic Inferences for the PKA Cα/Cβ Family in the Chordate Lineage
The well-resolved phylogenetic tree in Fig. 2 strongly suggests the following sequence of events during the evolution of the PKA Cα/Cβ gene family: a single PKA Cα/Cβ-like gene in the common ancestor of chordates, arthropods and echinoderms was duplicated in a common ancestor of vertebrates, which lead to the two paralogous genes corresponding to PKA Cα and Cβ. These two genes were again duplicated in a common ancestor of teleost fishes, leading to paralogs that we suggest are denoted PKA Cα-I, Cα-II, Cβ-I, and Cβ-II.
The data indicates that the first PKA Cα/Cβ gene duplication, resulting in PKA Cα and PKA Cβ, took place after the divergence of the urochordate and cephalochordate lineages. Currently there are only fragments of PKA Cα/Cβ homologs available in public databases for the chondrichthyes (dogfish shark and little skate) and the cyclostome sea lamprey (Table S1), but the PKA Cα/Cβ homologs also in these organisms appear to occur in pairs. Unfortunately, the phylogenetic signal in the data is too weak to classify these sequences as PKA Cα or PKA Cβ, and to exclude the possibility that these paralogous gene pairs are results of independent gene duplications, but the most parsimonious explanation for this distribution of PKA Cα/Cβ homologs is that a single PKA Cα/Cβ gene duplication occurred before the divergence of the jawless fish lineage and the subsequent divergence of sharks and skates. Interestingly, this timing of the PKA Cα/Cβ gene duplication coincides with the two rounds (2R) of whole genome duplication (2R hypothesis) that took place after the emergence of the invertebrate chordates and before the radiation of jawed vertebrates [62]–[64]. The PKA Cα and PKA Cβ gene split is thus likely to have occurred in the Cambrian, roughly 500 Mya [65], [66]. Canaves and Taylor [38] found that the gene duplications of the PKA regulatory subunits resulting in the paralogs RIα and RIβ as well as RIIα and RIIβ also occurred in the chordate lineage, suggesting that the gene duplications of the R and C subunits might have taken place simultaneously.
The secondary duplications of PKA Cα and PKA Cβ (Figs. 2 and 3) appear to be unique to teleost fishes, and might have coincided with the teleost whole genome duplication that took place 226–316 Mya [67], [68]. Finally, the presence of two X. laevis PKA Cα paralogs and a single X. tropicalis PKA Cα (Fig. 3A) in the amphibian genomes is consistent with the recent whole genome duplication event in the common ancestor of the X. laevis group not found in X. tropicalis [69], [70].
As expected, both the subtrees for PKA Cα (Fig. 3A) and PKA Cβ (Fig. 3B) have eutherian clades with the marsupial orthologs as sister clades, and with Sauropsida (birds and reptiles, Fig. 3B only) and frogs appearing as sister clades deeper into the phylogenetic tree. We were unable to find the PKA Cα gene in any of the released genomes of Sauropsida, and this clade is consequently missing in Fig. 3A. However, a single EST (expressed sequence tag) sequence from a chicken testis library (GenBank identifier CN229123 [71]) appears to confirm the presence of PKA Cα also in birds. Several vertebrate species are missing either PKA Cα or PKA Cβ in the current genomic data sets, but this is most likely due to low sequence coverage in unfinished genomes. We find no evidence for extensive PKA Cα or Cβ gene loss in any of the main vertebrate groups.
The marsupial M. domestica PKA Cβ appears to be particularly fast-evolving (Fig. 3B). The M. eugenii PKA Cβ ortholog is present in the genome, but the full sequence is currently unknown. Both marsupial genomes [72], [73] have two PKA Cα paralogs. Marsupial PKA Cα-I has the same exon/intron structure as PKA Cα in all other mammals. Marsupial PKA Cα-II, however, is intronless and a putative PKA Cα retroposon (Fig. 3A). The full-length sequences are not at present available for all the marsupial PKA Cα homologs, but interestingly, intronless PKA Cα-II appears to be significantly more conserved than the ancestral variant PKA Cα-I. The 5′ segment of the two marsupial PKA Cα-II orthologs (only the 232 5′ codons are available in the sequence for M. eugenii) have 17 synonymous and no non-synonymous mutations, suggesting strong purifying selection. For PKA Cα-I, the 294 codons corresponding to exons 2–9 (exons 1 and 10 are missing in the current M. eugenii genomic sequence) have 70 mutations, including a 3 nucleotide insertion in opossum PKA Cα-I, and 21 of these are non-synonymous. These data, although limited, strongly suggests that the retroposon PKA Cα-II have become functional in marsupials and that the purifying selection acting upon PKA Cα-I and PKA Cβ has become less stringent.
Vertebrate PKA Cα and Cβ Mainly Differs in the C-tail and in Subdomains I and II
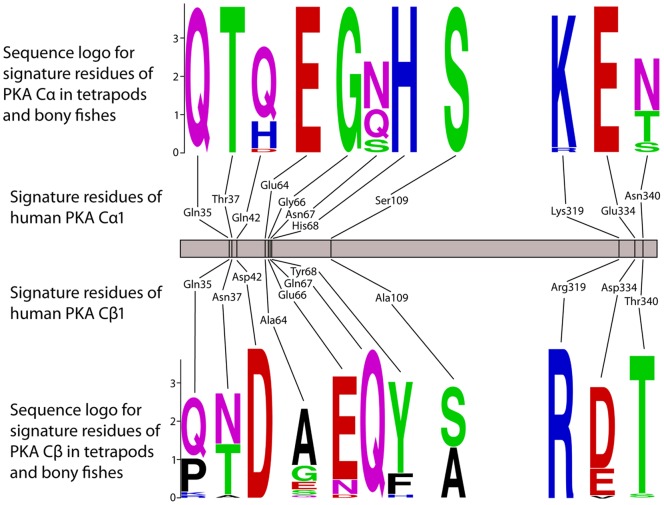
In order to elucidate the potential differences between the PKA Cα and PKA Cβ protein subfamilies, an MSA of all available vertebrate homologs was generated. In this set of 27 PKA Cα and 33 PKA Cβ, there are within the 335 sites/columns of the Core16–350 only 62 sites (19%), that are phylogenetically informative while 235 sites are fully conserved in all taxa, again reflecting the very high degree of purifying selection. A manual inspection of the MSA showed that eleven sites/columns could tentatively be used to discriminate between the two PKA subfamilies. Sequence logos for these eleven sites are shown in Fig. 4. Human PKA Cα1 residues Gln35, Thr37, Glu64, Gly66, His68, Ser109, and Glu334 are fully conserved in all vertebrate PKA Cα, while at the corresponding sites in PKA Cβ the sequence conservation is less stringent. Similarly, human PKA Cβ1 residues Asp42, Gln67, Arg319 are fully conserved in vertebrate PKA Cβ (Fig. 4). The single site where there is no overlap between amino acid use in the two subfamilies is at residues 66 where PKA Cα and Cβ have Gly and Glu/Asn/Asp, respectively.
Figure 4. The identity of eleven amino acids in the protein chain may define the Cα and Cβ branches of PKA catalytic subunits.
Our full set of PKA catalytic subunits (Materials and Methods S1) from bony fishes and tetrapods, comprising 27 Cα and 33 Cβ, was employed to identify eleven amino acid positions that together may be used to classify a PKA catalytic subunit as belonging to one of the two branches. The sequence logos define the PKA Cα and Cβ clades within the Teleostomi, which includes the familiar classes of bony fishes, birds, mammals, reptiles, and amphibians. We find invariable Gln35, Thr37, Glu64, Gly66, His68, Ser109 and Glu334 in Cα and invariable Asp42, Gln67, and Arg319 in Cβ (Cα1/Cβ1 numbering). The residues in the corresponding positions in human Cα1 and Cβ1 are also shown.
The residues that correspond to the eleven sites that tentatively discriminates between the subfamilies PKA Cα and Cβ are all exposed at the protein surface, in or close to loop structures, in subdomains I and II and in the C-tail (Fig. 5). None of these residues are located close to the kinase active site and their identity is not likely to affect PKA kinase activity. Likewise, they are not located near the protein surface segments that are known to interact with the PKA regulatory subunits (Fig. 5B), and these residues should not be important for R subunit interactions. Several of the eleven residues, especially residues 64, 319, 334 and 340, are protruding their side chains into the solvent and might be targets for PKA Cα- or PKA Cβ-specific post-translational modifications and/or protein-protein interactions. Particularly, Glu64 is conserved in all vertebrate PKA Cα, while residue 64 is variable in 33 Cβ, being Ala in all mammals and Glu in only three fish homologs. In PKA Cβ Glu66 is absolutely conserved, except in five of the fish homologs, while this residue is conserved as Gly in Cα.
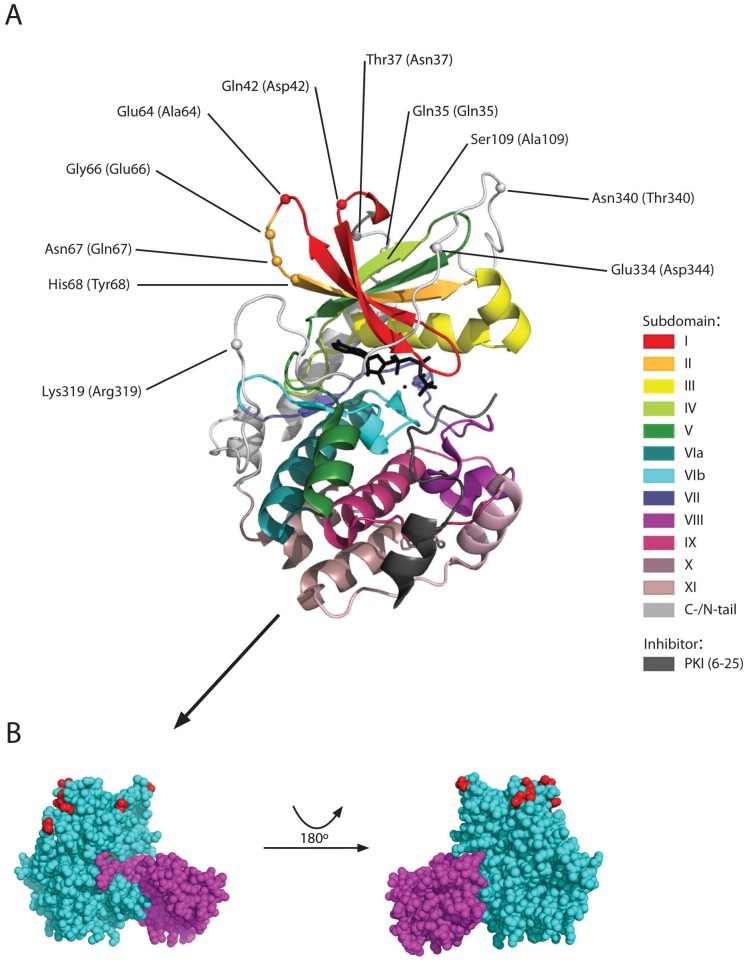
Figure 5. Signature residues defining PKA Cα and Cβ do not interact with ATP, peptide inhibitor PKIα or the kinase regulatory subunit.
A The tentative signature residues of PKA Cα and Cβ (Fig. 4) are highlighted in a structural model of PKA Cα1 in complex with a truncated PKIα (residues 6–25). Signature amino acids in human Cα1 and Cβ1 are shown without and within parenthesis, respectively. The conserved kinase core has been divided into subdomains (represented in different colors) [87] as defined by Hanks and Hunter [88]. ATP is rendered as sticks (black) and two divalent cations as black spheres. The model is based on the experimental structure of Thompson et al. [56] (PDB identifier 3FJQ). B Residues in Cα1 (cyan) interacting with regulatory subunit RIα (purple, residues 92–245 of bovine RIα only) are mainly restricted to the large lobe and do not overlap with any of the Cα signature residues (red). The complex is shown with the same orientation of Cα1 as in panel A (left) and rotated 180° (right). The model is based on the experimental structure of Kim et al. [5] (PDB identifier 3FHI).
Strong Purifying Selection is Lost in PKA Cγ
A comparison of PKA Cα1 from human and the galago, a prosimian primate, shows that there are 59 and 4 synonymous (silent) and non-synonymous (amino acid changing) mutations, respectively. Between human and macaque PKA Cγ there are slightly fewer, 44, mutations, but from these 31 amino acid changes, indicating that the strong purifying selection in the PKA Cα lineage is lost in PKA Cγ. A powerful tool for evaluating the evolution of protein coding sequences, is calculating the ratio of the non-synonymous (Ka) and synonymous (Ks) substitution rates, where Ks is the number of synonymous substitutions per synonymous site and Ka is the number of non-synonymous substitutions per non-synonymous site [74]. A Ka/Ks <1 indicates purifying (negative) selection, Ka/Ks >1 is a sign of positive selection, while Ka/Ks ∼ 1 indicates neutral evolution of the protein. Table 1 shows Ka/Ks for comparisons of five representative tetrapod PKA Cα homologs and five primate PKA Cγ homologs. Due to missing sequences in the databases it was not possible to compare the same species for Cα and Cγ. For the PKA Cα sequences, the average Ka/Ks is 0.011. PKA Cα sequences were also compared for other placental mammals and Ka/Ks were found to be in the range 0.004–0.023. Ka/Ks for a comparison of human PKA Cα and Cβ, and of the human and mouse PKA Cβ orthologs, were 0.0076 and 0.0198, respectively. These data for the PKA Cα/Cβ homologs in placental mammals again confirms the very strong purifying selection acting upon these kinases.
Table 1. Ratio of amino acid replacing (Ka) and silent (Ks) mutation rates for all pairwise comparisons of five tetrapod PKA Cα homologs and five primate PKA Cγ homologs.
| Ka/Ksa | ||||
| H. sapiens Cα | O. garnettii Cα | M. musculus Cα | B. taurus Cα | |
| O. garnettii Cα | 0.0159 (0.0158) | |||
| M. musculus Cα | 0.0141 (0.0141) | 0.0230 (0.0226) | ||
| B. taurus Cα | 0.0064 (0.0063) | 0.0116 (0.0111) | 0.0156 (0.0158) | |
| X. tropicalis Cα | 0.0065 (0.0060) | 0.0055 (0.0056) | 0.0065 (0.0064) | 0.0059 (0.0060) |
| K a/ K s b | ||||
| H. sapiens Cγ | P. troglodytes Cγ | P. pygmaeus Cγ | G. gorilla Cγ | |
| P. troglodytes Cγ | 0.135 (0.127) | |||
| P. pygmaeus Cγ | 0.290 (0.324) | 0.175 (0.194) | ||
| G. gorilla Cγ | 1.360 (1.207) | 0.162 (0.148) | 0.295 (0.319) | |
| M. mulatta Cγ | 0.697 (0.678) | 0.419 (0.406) | 0.393 (0.393) | 0.621 (0.603) |
Ka/Ks ratios for pairwise comparisons of PKA Cα from human, a prosimian primate (O. garnettii), mouse (M. musculus), cattle (B. taurus) and a frog (X. tropicalis) calculated according to the model of Goldman and Yang [89] and the model averaging method of Zhang et al. (in parenthesis) [90] for the Core16–350 sequence segment.
Ka/Ks ratios for pairwise comparisons of PKA Cγ from human, chimpanzee (P. troglodytes), orangutan (P. pygmaeus), gorilla (G. gorilla) and rhesus macaque (M. mulatta) calculated as described above.
The Ka/Ks values from the comparison of the primate PKA Cγ sequences in Table 1 are in the range 0.14–1.36. Due to the fairly close evolutionary relationships between these species, the number of mutations in PRKACG transcripts between these species is rather low, for example 9, 31 and 46 between human and chimpanzee, orangutan, and macaque, respectively. Consequently, the Ka/Ks values are not expected to be highly reliable, but the average value of 0.45 clearly suggests that the strong purifying selection in the PKA Cα lineage is lost in PKA Cγ. This finding that mutations in PKA Cγ appear to be neutral, combined with the loss of a functional Cγ in gibbons and marmoset (vide supra), suggest that there are no evolutionary constraints on maintaining a functional PKA Cγ protein in higher primates. However, this analysis does not exclude the possibility that the PKA Cγ transcript has an important function in humans, great apes and other Simiiformes, for example in regulation of PKA Cα/Cβ transcript processing [75].
Conclusion
We have shown that the PKA Cα and Cβ catalytic subunits found in chordates and other animal species builds a phylogenetic clade of kinases with a very high degree of conservation at the protein level. In the core segment corresponding to exons 2–10 of vertebrate Cα1/Cβ1 the synonymous mutation rate is approximately two orders of magnitude larger than the amino acid changing mutation rate. All the main residues and sequence segments previously shown to be important for human Cα/Cβ function (Fig. 1C), including the phosphorylation sites, the ATP and Mg2+ interacting residues and the DFG and P+1 motifs, are basically fully conserved in all homologs in chordates, insects and other animal sequences investigated in the current study. The few residues that differ in Cα and Cβ (Fig. 4) should be investigated in order to elucidate possible functional differences between the two paralogs. Finally, the Cα1-derived expressed retroposon Cγ found in higher primates appears to be evolving neutrally and appears to have no function as a mature protein.
Supporting Information
Multiple sequence alignment of segment corresponding to exons 2–10 of human PKA Cα1 ( i.e. residues 16–350). All sequences are described in Materials and Methods S1. Residue numbering of human PKA Cα1 (identifier P17612) is shown above the sequence. Sequences belonging to the Cα, Cγ, and Cβ clades are marked at the right by a green, blue, and red bar, respectively. The figure was prepared with Jalview [44].
(PDF)
Sequence data for PKA catalytic subunit homologs from chordates collected and manipulated as described in Materials and Methods S1.
(PDF)
Details on sequence data collection.
(PDF)
Acknowledgments
We are grateful to Russell Orr and Kamran Shalchian-Tabrizi for help and useful comments and the University of Oslo Bioportal for computational infrastructure.
Funding Statement
The project was funded by the Research Council of Norway. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S (2002) The protein kinase complement of the human genome. Science 298: 1912–1934. [DOI] [PubMed] [Google Scholar]
- 2. Skålhegg BS, Taskén K (2000) Specificity in the cAMP/PKA signaling pathway. Differential expression, regulation, and subcellular localization of subunits of PKA. Front Biosci 5: D678–D693. [DOI] [PubMed] [Google Scholar]
- 3. Taylor SS, Yang J, Wu J, Haste NM, Radzio-Andzelm E, et al. (2004) PKA: a portrait of protein kinase dynamics. Biochim Biophys Acta 1697: 259–269. [DOI] [PubMed] [Google Scholar]
- 4. Krebs EG, Beavo JA (1979) Phosphorylation-dephosphorylation of enzymes. Annu Rev Biochem 48: 923–959. [DOI] [PubMed] [Google Scholar]
- 5. Kim C, Xuong NH, Taylor SS (2005) Crystal structure of a complex between the catalytic and regulatory (RIα) subunits of PKA. Science 307: 690–696. [DOI] [PubMed] [Google Scholar]
- 6. Anand GS, Krishnamurthy S, Bishnoi T, Kornev A, Taylor SS, et al. (2010) Cyclic AMP- and (R p)-cAMPS-induced conformational changes in a complex of the catalytic and regulatory (RIα) subunits of cyclic AMP-dependent protein kinase. Mol Cell Proteomics 9: 2225–2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sjoberg TJ, Kornev AP, Taylor SS (2010) Dissecting the cAMP-inducible allosteric switch in protein kinase A RIα. Protein Sci 19: 1213–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Taskén K, Skålhegg BS, Taskén KA, Solberg R, Knutsen HK, et al. (1997) Structure, function, and regulation of human cAMP-dependent protein kinases. Adv Second Messenger Phosphoprotein Res 31: 191–204. [DOI] [PubMed] [Google Scholar]
- 9. Corbin JD, Keely SL, Park CR (1975) The distribution and dissociation of cyclic adenosine 3′: 5′-monophosphate-dependent protein kinases in adipose, cardiac, and other tissues. J Biol Chem 250: 218–225. [PubMed] [Google Scholar]
- 10. Døskeland SO, Ogreid D (1981) Binding proteins for cyclic AMP in mammalian tissues. Int J Biochem 13: 1–19. [DOI] [PubMed] [Google Scholar]
- 11. Dell’Acqua ML, Scott JD (1997) Protein kinase A anchoring. J Biol Chem 272: 12881–12884. [DOI] [PubMed] [Google Scholar]
- 12. Beene DL, Scott JD (2007) A-kinase anchoring proteins take shape. Curr Opin Cell Biol 19: 192–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Colledge M, Scott JD (1999) AKAPs: from structure to function. Trends Cell Biol 9: 216–221. [DOI] [PubMed] [Google Scholar]
- 14. Wang L, Sunahara RK, Krumins A, Perkins G, Crochiere ML, et al. (2001) Cloning and mitochondrial localization of full-length D-AKAP2, a protein kinase A anchoring protein. Proc Natl Acad Sci U S A 98: 3220–3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Taskén K, Aandahl EM (2004) Localized effects of cAMP mediated by distinct routes of protein kinase A. Physiol Rev. 84: 137–167. [DOI] [PubMed] [Google Scholar]
- 16. Jarnaess E, Taskén K (2007) Spatiotemporal control of cAMP signalling processes by anchored signalling complexes. Biochem Soc Trans 35: 931–937. [DOI] [PubMed] [Google Scholar]
- 17. Beebe SJ, Øyen O, Sandberg M, Frøysa A, Hansson V, et al. (1990) Molecular cloning of a tissue-specific protein kinase (Cγ) from human testis - representing a third isoform for the catalytic subunit of cAMP-dependent protein kinase. Mol Endocrinol 4: 465–475. [DOI] [PubMed] [Google Scholar]
- 18. Schiebel K, Winkelmann M, Mertz A, Xu X, Page DC, et al. (1997) Abnormal XY interchange between a novel isolated protein kinase gene, PRKY, and its homologue, PRKX, accounts for one third of all (Y+)XX males and (Y−)XY females. Hum Mol Genet 6: 1985–1989. [DOI] [PubMed] [Google Scholar]
- 19. Zimmermann B, Chiorini JA, Ma Y, Kotin RM, Herberg FW (1999) PrKX is a novel catalytic subunit of the cAMP-dependent protein kinase regulated by the regulatory subunit type I. J Biol Chem. 274: 5370–5378. [DOI] [PubMed] [Google Scholar]
- 20. Pearce LR, Komander D, Alessi DR (2010) The nuts and bolts of AGC protein kinases. Nat Rev Mol Cell Biol 11: 9–22. [DOI] [PubMed] [Google Scholar]
- 21. Showers MO, Maurer RA (1988) Cloning of cDNA for the catalytic subunit of cAMP-dependent protein kinase. Methods Enzymol 159: 311–318. [DOI] [PubMed] [Google Scholar]
- 22. San Agustin JT, Leszyk JD, Nuwaysir LM, Witman GB (1998) The catalytic subunit of the cAMP-dependent protein kinase of ovine sperm flagella has a unique amino-terminal sequence. J Biol Chem 273: 24874–24883. [DOI] [PubMed] [Google Scholar]
- 23. Reinton N, Ørstavik S, Haugen TB, Jahnsen T, Taskén K, et al. (2000) A novel isoform of human cyclic 3′,5′-adenosine monophosphate-dependent protein kinase, Cα-s, localizes to sperm midpiece. Biol Reprod 63: 607–611. [DOI] [PubMed] [Google Scholar]
- 24. San Agustin JT, Witman GB (2001) Differential expression of the Cs and Cα1 isoforms of the catalytic subunit of cyclic 3′,5′-adenosine monophosphate-dependent protein kinase in testicular cells. Biol Reprod 65: 151–164. [DOI] [PubMed] [Google Scholar]
- 25. Desseyn JL, Burton KA, McKnight GS (2000) Expression of a nonmyristylated variant of the catalytic subunit of protein kinase A during male germ-cell development. Proc Natl Acad Sci U S A 97: 6433–6438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Skålhegg BS, Huang Y, Su T, Idzerda RL, McKnight GS, et al. (2002) Mutation of the Cα subunit of PKA leads to growth retardation and sperm dysfunction. Mol Endocrinol 16: 630–639. [DOI] [PubMed] [Google Scholar]
- 27. Nolan MA, Babcock DF, Wennemuth G, Brown W, Burton KA, et al. (2004) Sperm-specific protein kinase A catalytic subunit Cα2 orchestrates cAMP signaling for male fertility. Proc Natl Acad Sci U S A 101: 13483–13488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Uhler MD, Chrivia JC, McKnight GS (1986) Evidence for a second isoform of the catalytic subunit of cAMP-dependent protein kinase. J Biol Chem 261: 15360–15363. [PubMed] [Google Scholar]
- 29. Wiemann S, Kinzel V, Pyerin W (1991) Isoform Cβ2, an unusual form of the bovine catalytic subunit of cAMP-dependent protein kinase. J Biol Chem 266: 5140–5146. [PubMed] [Google Scholar]
- 30. Guthrie CR, Skålhegg BS, McKnight GS (1997) Two novel brain-specific splice variants of the murine Cβ gene of cAMP-dependent protein kinase. J Biol Chem 272: 29560–29565. [DOI] [PubMed] [Google Scholar]
- 31. Ørstavik S, Reinton N, Frengen E, Langeland BT, Jahnsen T, et al. (2001) Identification of novel splice variants of the human catalytic subunit Cβ of cAMP-dependent protein kinase. Eur J Biochem 268: 5066–5073. [DOI] [PubMed] [Google Scholar]
- 32. Kvissel AK, Ørstavik S, Øistad P, Rootwelt T, Jahnsen T, et al. (2004) Induction of Cβ splice variants and formation of novel forms of protein kinase A type II holoenzymes during retinoic acid-induced differentiation of human NT2 cells. Cell Signal 16: 577–587. [DOI] [PubMed] [Google Scholar]
- 33. Funderud A, Henanger HH, Hafte TT, Amieux PS, Ørstavik S, et al. (2006) Identification, cloning and characterization of a novel 47 kDa murine PKA C subunit homologous to human and bovine Cβ2. BMC Biochem 7: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Larsen AC, Kvissel AK, Hafte TT, Avellan CI, Eikvar S, et al. (2008) Inactive forms of the catalytic subunit of protein kinase A are expressed in the brain of higher primates. FEBS J 275: 250–262. [DOI] [PubMed] [Google Scholar]
- 35. Reinton N, Haugen TB, Ørstavik S, Skålhegg BS, Hansson V, et al. (1998) The gene encoding the Cγ catalytic subunit of cAMP-dependent protein kinase is a transcribed retroposon. Genomics 49: 290–297. [DOI] [PubMed] [Google Scholar]
- 36. Beebe SJ, Salomonsky P, Jahnsen T, Li Y (1992) The Cγ subunit is a unique isozyme of the cAMP-dependent protein kinase. J Biol Chem 267: 25505–25512. [PubMed] [Google Scholar]
- 37. Zhang W, Morris GZ, Beebe SJ (2004) Characterization of the cAMP-dependent protein kinase catalytic subunit Cγ expressed and purified from sf9 cells. Protein Expr Purif 35: 156–169. [DOI] [PubMed] [Google Scholar]
- 39. The UniProt Consortium (2011) Ongoing and future developments at the Universal Protein Resource. Nucleic Acids Res 39: D214–D219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sayers EW, Barrett T, Benson DA, Bolton E, Bryant SH, et al. (2011) Database resources of the National Center for Biotechnology Information. Nucleic Acids Res 39: D38–D51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Flicek P, Amode MR, Barrell D, Beal K, Brent S, et al. (2011) Ensembl 2011. Nucleic Acids Res 39: D800–D806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215: 403–410. [DOI] [PubMed] [Google Scholar]
- 43. Edgar RC (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32: 1792–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Waterhouse AM, Procter JB, Martin DMA, Clamp M, Barton GJ (2009) Jalview Version 2 - a multiple sequence alignment editor and analysis workbench. Bioinformatics 25: 1189–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Posada D, Crandall KA (1998) MODELTEST: testing the model of DNA substitution. Bioinformatics 14: 817–818. [DOI] [PubMed] [Google Scholar]
- 46. Abascal F, Zardoya R, Posada D (2005) ProtTest: selection of best-fit models of protein evolution. Bioinformatics 21: 2104–2105. [DOI] [PubMed] [Google Scholar]
- 47. Le SQ, Gascuel O (2008) An improved general amino acid replacement matrix. Mol Biol Evol 25: 1307–1320. [DOI] [PubMed] [Google Scholar]
- 48. Jones DT, Taylor WR, Thornton JM (1992) The rapid generation of mutation data matrices from protein sequences. Comput Appl Biosci 8: 275–282. [DOI] [PubMed] [Google Scholar]
- 49. Huelsenbeck JP, Ronquist F (2001) MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 17: 754–755. [DOI] [PubMed] [Google Scholar]
- 50. Ronquist F, Huelsenbeck JP (2003) MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19: 1572–1574. [DOI] [PubMed] [Google Scholar]
- 51. Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, et al. (2010) New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol 59: 307–321. [DOI] [PubMed] [Google Scholar]
- 52. Stamatakis A (2006) RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22: 2688–2690. [DOI] [PubMed] [Google Scholar]
- 53. Huson DH, Richter DC, Rausch C, Dezulian T, Franz M, et al. (2007) Dendroscope: An interactive viewer for large phylogenetic trees. BMC Bioinformatics 8: 460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Crooks GE, Hon G, Chandonia JM, Brenner SE (2004) WebLogo: a sequence logo generator. Genome Res 14: 1188–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wang D, Zhang Y, Zhang Z, Zhu J, Yu J (2010) KaKs_Calculator 2.0: a toolkit incorporating gamma-series methods and sliding window strategies. Genomics Proteomics Bioinformatics 8: 77–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Thompson EE, Kornev AP, Kannan N, Kim C, Ten Eyck LF, et al. (2009) Comparative surface geometry of the protein kinase family. Protein Sci 18: 2016–2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Whelan S, Lio P, Goldman N (2001) Molecular phylogenetics: state-of-the-art methods for looking into the past. Trends Genet 17: 262–272. [DOI] [PubMed] [Google Scholar]
- 58.Felsenstein J (2004) Inferring Phylogenies. Sunderland, MA: Sinauer Associates.
- 59. Jeffroy O, Brinkmann H, Delsuc F, Philippe H (2006) Phylogenomics: the beginning of incongruence? Trends Genet 22: 225–231. [DOI] [PubMed] [Google Scholar]
- 60. Bergsten J (2005) A review of long-branch attraction. Cladistics 21: 163–193. [DOI] [PubMed] [Google Scholar]
- 61. Delsuc F, Brinkmann H, Chourrout D, Philippe H (2006) Tunicates and not cephalochordates are the closest living relatives of vertebrates. Nature 439: 965–968. [DOI] [PubMed] [Google Scholar]
- 62. Dehal P, Boore JL (2005) Two rounds of whole genome duplication in the ancestral vertebrate. PLoS Biol 3: e314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Masanori K (2007) The 2R hypothesis: an update. Curr Opin Immunol 19: 547–552. [DOI] [PubMed] [Google Scholar]
- 64. Putnam NH, Butts T, Ferrier DE, Furlong RF, Hellsten U, et al. (2008) The amphioxus genome and the evolution of the chordate karyotype. Nature 453: 1064–1071. [DOI] [PubMed] [Google Scholar]
- 65. Kuraku S, Meyer A, Kuratani S (2009) Timing of genome duplications relative to the origin of the vertebrates: did cyclostomes diverge before or after? Mol Biol Evol 26: 47–59. [DOI] [PubMed] [Google Scholar]
- 66. Van de Peer Y, Maere S, Meyer A (2009) The evolutionary significance of ancient genome duplications. Nat Rev Genet 10: 725–732. [DOI] [PubMed] [Google Scholar]
- 67. Meyer A, Van de Peer Y (2005) From 2R to 3R: evidence for a fish-specific genome duplication (FSGD). Bioessays 27: 937–945. [DOI] [PubMed] [Google Scholar]
- 68. Hurley IA, Mueller RL, Dunn KA, Schmidt EJ, Friedman M, et al. (2007) A new time-scale for ray-finned fish evolution. Proc Biol Sci 274: 489–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Bisbee CA, Baker MA, Wilson AC, Haji-Azimi I, Fischberg M (1977) Albumin phylogeny for clawed frogs (Xenopus). Science 195: 785–787. [DOI] [PubMed] [Google Scholar]
- 70. Hellsten U, Harland RM, Gilchrist MJ, Hendrix D, Jurka J, et al. (2010) The genome of the Western clawed frog Xenopus tropicalis . Science 328: 633–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Savolainen P, Fitzsimmons C, Arvestad L, Andersson L, Lundeberg J (2005) ESTs from brain and testis of White Leghorn and red junglefowl: annotation, bioinformatic classification of unknown transcripts and analysis of expression levels. Cytogenet Genome Res 111: 79–87. [DOI] [PubMed] [Google Scholar]
- 72. Mikkelsen TS, Wakefield MJ, Aken B, Amemiya CT, Chang JL, et al. (2007) Genome of the marsupial Monodelphis domestica reveals innovation in non-coding sequences. Nature 447: 167–177. [DOI] [PubMed] [Google Scholar]
- 73. Renfree MB, Papenfuss AT, Deakin JE, Lindsay J, Heider T, et al. (2011) Genome sequence of an Australian kangaroo, Macropus eugenii, provides insight into the evolution of mammalian reproduction and development. Genome Biol 12: R81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Hurst LD (2002) The Ka/Ks ratio: diagnosing the form of sequence evolution. Trends Genet 18: 486. [DOI] [PubMed] [Google Scholar]
- 75. Pink RC, Wicks K, Caley DP, Punch EK, Jacobs L, et al. (2011) Pseudogenes: pseudo-functional or key regulators in health and disease? RNA 17: 792–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Knighton DR, Zheng JH, Ten Eyck LF, Ashford VA, Xuong NH, et al. (1991) Crystal structure of the catalytic subunit of cyclic adenosine monophosphate-dependent protein kinase. Science 253: 407–414. [DOI] [PubMed] [Google Scholar]
- 77. Breitenlechner C, Engh RA, Huber R, Kinzel V, Bossemeyer D, et al. (2004) The typically disordered N-terminus of PKA can fold as a helix and project the myristoylation site into solution. Biochemistry 43: 7743–7749. [DOI] [PubMed] [Google Scholar]
- 78. Seifert MH, Breitenlechner CB, Bossemeyer D, Huber R, Holak TA, et al. (2002) Phosphorylation and flexibility of cyclic-AMP-dependent protein kinase (PKA) using 31P NMR spectroscopy. Biochemistry 41: 5968–5977. [DOI] [PubMed] [Google Scholar]
- 79. Gesellchen F, Bertinetti O, Herberg FW (2006) Analysis of posttranslational modifications exemplified using protein kinase A. Biochim Biophys Acta. 1764: 1788–1800. [DOI] [PubMed] [Google Scholar]
- 80. Torkamani A, Kannan N, Taylor SS, Schork NJ (2008) Congenital disease SNPs target lineage specific structural elements in protein kinases. Proc Natl Acad Sci U S A 105: 9011–9016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Khavrutskii IV, Grant B, Taylor SS, McCammon JA (2009) A transition path ensemble study reveals a linchpin role for Mg2+ during rate-limiting ADP release from protein kinase A. Biochemistry. 48: 11532–11545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Yang J, Kennedy EJ, Wu J, Deal MS, Pennypacker J, et al. (2009) Contribution of non-catalytic core residues to activity and regulation in protein kinase A. J Biol Chem. 284: 6241–6248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Kornev AP, Haste NM, Taylor SS, Eyck LF (2006) Surface comparison of active and inactive protein kinases identifies a conserved activation mechanism. Proc Natl Acad Sci U S A 103: 17783–17788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Badrinarayan P, Sastry GN (2011) Sequence, structure, and active site analyses of p38 MAP kinase: exploiting DFG-out conformation as a strategy to design new type II leads. J Chem Inf Model 51: 115–129. [DOI] [PubMed] [Google Scholar]
- 85. Taylor SS, Kim C, Cheng CY, Brown SH, Wu J, et al. (2008) Signaling through cAMP and cAMP-dependent protein kinase: diverse strategies for drug design. Biochim Biophys Acta 1784: 16–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Wu J, Brown SH, von Daake S, Taylor SS (2007) PKA type IIα holoenzyme reveals a combinatorial strategy for isoform diversity. Science 318: 274–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Niedner RH, Buzko OV, Haste NM, Taylor A, Gribskov M, et al. (2006) Protein kinase resource: an integrated environment for phosphorylation research. Proteins 63: 78–86. [DOI] [PubMed] [Google Scholar]
- 88. Hanks SK, Hunter T (1995) Protein kinases 6. The eukaryotic protein kinase superfamily: kinase (catalytic) domain structure and classification. FASEB J 9: 576–596. [PubMed] [Google Scholar]
- 89. Goldman N, Yang Z (1994) A codon-based model of nucleotide substitution for protein-coding DNA sequences. Mol Biol Evol 11: 725–736. [DOI] [PubMed] [Google Scholar]
- 90. Zhang Z, Li J, Zhao XQ, Wang J, Wong GKS, et al. (2006) KaKs_Calculator: calculating Ka and Ks through model selection and model averaging. Genomics Proteomics Bioinformatics 4: 259–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Multiple sequence alignment of segment corresponding to exons 2–10 of human PKA Cα1 ( i.e. residues 16–350). All sequences are described in Materials and Methods S1. Residue numbering of human PKA Cα1 (identifier P17612) is shown above the sequence. Sequences belonging to the Cα, Cγ, and Cβ clades are marked at the right by a green, blue, and red bar, respectively. The figure was prepared with Jalview [44].
(PDF)
Sequence data for PKA catalytic subunit homologs from chordates collected and manipulated as described in Materials and Methods S1.
(PDF)
Details on sequence data collection.
(PDF)