The gaseous hormone ethylene (ET) controls diverse aspects of plant life (1). In innate immunity, ET is often associated with resistance to necrotrophic pathogens (2). ET also plays a role in induced systemic resistance that is triggered by the perception of beneficial microbes in the rhizosphere (2). ET is often antagonistic with the hormones salicylic acid and jasmonic acid (2). However, perception of pathogen-associated molecular patterns (PAMPs) by plant pattern-recognition receptors (PRRs) leads to the production of ET, salicylic acid, and jasmonic acid, and all three hormones are required for local PAMP-induced resistance to pathogens (3, 4). The precise role of ET in PAMP-triggered immunity (PTI) was therefore unclear. In PNAS, Tintor et al. and Liu et al. (5, 6) present convincing evidence for a role of ET in an amplification loop required for sustained PTI, and some growth responses triggered by the hormone.
The best-studied PRRs are the Arabidopsis leucine-rich repeat receptor kinases (LRR-RKs) FLS2 and EFR that perceive bacterial flagellin (or flg22) and EF-Tu (or elf18), respectively (7). Following ligand-binding, FLS2 and EFR recruit the regulatory LRR-RK BAK1 leading to transphosphorylation events between these proteins, as well as with the cytoplasmic kinase BIK1 (7–9).
Previous genetic analyses revealed that ET perception and signaling are required for proper FLS2 expression (10, 11). Indeed, the ET-activated transcription factors EIN3 and EIL1 bind the FLS2 promoter to regulate FLS2 transcription (10). This finding suggests that flg22-induced ET production enables amplification and maintenance of PTI by replenishment of FLS2 at the plasma membrane, which is otherwise degraded following flg22 perception (12). In a forward genetic screen for elf18-insensitive mutants, Tintor et al. (5) identified a unique allele of the ER-localized protein EIN2, which is the central regulator of ET signaling (13). Notably, although they could confirm that EIN2 is required for proper FLS2 expression, no effect on the transcript accumulation of EFR could be observed in ein2 mutants, suggesting that ET could play an additional role. Specifically, the authors found that ET signaling is important for a subset of elf18-induced responses, such as the burst of reactive oxygen species (ROS), transcriptional reprogramming, callose deposition, but not MAP kinase activation. The key question that then arose was: how does ET regulate these responses?
A first hint was provided by the observation that PROPEP2 is among the elf18-induced genes that are regulated in an EIN2- and EIN3/EIL1-dependent manner (5). PROPEP2 is part of a seven-member family of propeptides that are proposed to act as damage-associated molecular patterns (14). PROPEP gene expression is regulated by diverse stimuli, and derived synthetic Pep peptides induce immune responses similar to those triggered by PAMPs (3, 15–19). Perception of Pep peptides depends on the related LRR-RKs PEPR1 and PEPR2 that show sequence and functional homologies with FLS2 and EFR (16, 20). Although PROPEP genes have been proposed to be part of an amplification loop for PTI, this hypothesis has never been tested directly. Tintor et al. (5) hypothesize that the expression of PROPEP2 and subsequent potential recognition by PEPR1/2 may contribute to certain responses triggered by elf18. Consistently, the elf18-induced expression of PR-1 (a late immune marker gene) was strongly reduced in the double-mutant pepr1 pepr2. Importantly, pepr1 pepr2 plants were also more susceptible to spray-infection with the hemibiotrophic bacterium Pseudomonas syringae pv. tomato (Pto) DC3000 and affected in the elf18-induced resistance to this bacterium.
The second hint came with the study from Liu et al. (6), which provides more mechanistic insights into the puzzle. Initially, the authors found the kinase domain of PEPR1 as a BIK1 interactor in a yeast two-hybrid screen. The authors could then confirm that full-length PEPR1 associates with BIK1 both in vitro and in vivo. Consistently, Liu et al. found that BIK1 is required for Pep1-induced immune responses and seedling growth inhibition. Per se, these findings are not surprising given that BIK1 also interacts with FLS2 and EFR and given the commonalities between the FLS2/EFR and PEPR1/2 pathways, in terms of the responses induced, association with, and genetic dependence on BAK1 (17, 18, 21, 22). However, some differences seem to exist between the perception systems. Although BIK1 and the related proteins PBL1, PBL2, and PBL5 are important for the flg22-induced ROS burst (6, 8, 9), only BIK1 and PBL1 interact with PEPR1 and play a role in Pep1-triggered ROS burst and callose deposition (6).
It was recently shown that BIK1 is phosphorylated in response to ET and is required for some responses triggered by ET, such as hypocotyl growth inhibition and wound-induced resistance to the necrotrophic fungus Botrytis cinerea (23). Given that ET regulates the expression of PROPEP1 and PROPEP2 (5, 14), Liu et al. (6) tested whether PEPR1/2 and BIK1 play a role in some ET-induced responses. The authors found that PEPR1/2 and BIK1 are indeed required for full expression of ET-induced genes and ET-triggered resistance to B. cinerea. Consistent with these and previous results (16, 20, 23), the authors could then connect ET, Pep1, PEPR1/2, and BIK1 by showing that PEPR1 and PEPR2 are required for both Pep1- and ET-induced BIK1 phosphorylation.
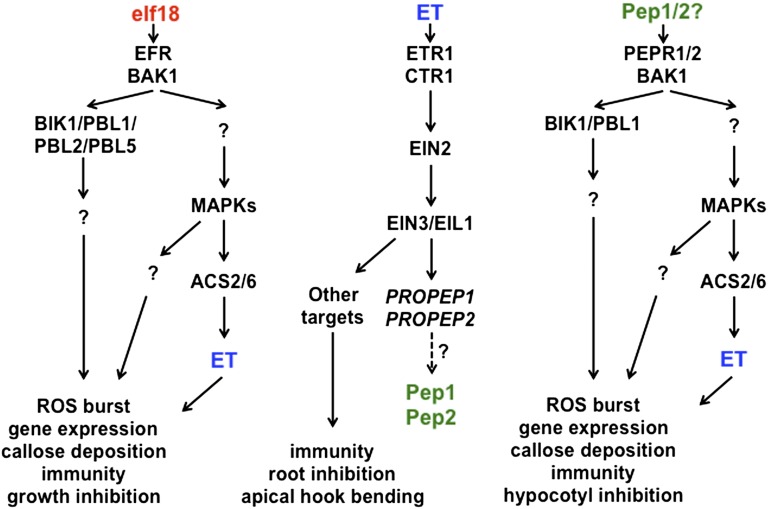
Taken together, the results from Tintor et al. and Liu et al. (5, 6) nicely illustrate the complexity of immune signaling (Fig. 1). These reports reveal that one function of PAMP-induced ET production, beyond the transcriptional control of some PRR-encoding genes, might be to induce the expression of PROPEP genes (such as PROPEP1 and PROPEP2). The corresponding putative Pep peptides are then recognized by PEPR1/2, leading to BIK1 phosphorylation and activation of a second wave of immune signaling. This PEPR1/2-mediated amplification loop plays an important role in ensuring optimal PAMP-induced late transcriptional reprogramming and immunity to both hemibiotrophic and necrotrophic pathogens.
Fig. 1.
A simplified model for the role of ET and PEPR1/2 in PAMP-triggered immunity and growth responses. PAMP perception (such as elf18 by EFR) triggers the production of ET. ET perception induces the expression of PROPEP genes. PROPEP-derived ligands are perceived by PEPR1/2 leading to BIK1 phosphorylation. Activation of the PEPR1/2 system leads to immune responses, including growth inhibition and sustained PAMP-induced responses.
Remarkably, Liu et al. (6) also found that PEPR1/2 are partially required for ET-induced hypocotyl growth inhibition in etiolated seedlings. This unexpected result suggests that the ET-induced PROPEP1 expression and potential subsequent Pep1 recognition by PEPR1/2 transduced by BIK1 underlies to some extent certain growth responses triggered by ET. These results illustrate that the hypocotyl growth inhibition of etiolated seedlings, which constitutes one of the hallmarks of the typical triple response triggered by ET perception (1), is indirect and most likely results from the activation of PEPR1/2-triggered immune responses leading to seedling growth inhibition, a response also observed upon treatment with the PAMPs flg22 and elf18 (3).
This model is, however, far from being so simple. Notably, an ET-independent contribution of PEPR1/2 to EFR-triggered immunity seems to exist, because elf18-induced PROPEP3 expression is not affected in ein2 seedlings. This ET-independent PEPR1/2-dependent function may operate as a backup mechanism if the ET signaling sector is lost. As such, these studies definitely confirm the previously hypothesized role of PEPR1/2 as an amplification system for PTI.
Moreover, an additional uncharacterized role for ET in EFR-triggered immunity that is PEPR1/2-independent must exist, as the pepr1 pepr2 and ein2 mutations have additive effects on the basal and elf18-induced immunity to Pto DC3000 (5). This notion is further supported by the fact that ein2 and pepr1 pepr2 plants are not necessarily affected in the same set of elf18-induced responses. For example, the elf18-induced ROS burst is PEPR1/2-independent (5). Similarly, flg22 induces a wild-type ROS burst in pepr1 pepr2 mutant plants (16), which seems to contradict the recently reported cross-dependency of the PEPR1/2 and FLS2 perception systems (19). Thus, in the same way that ET controls the expression of FLS2 (10, 11), it is possible that ET regulates the expression of additional immune components that act downstream of PRRs via the transcription factors EIN3/EIL1.
Despite these important advances, important questions remain to be answered. Most urgently, it is still unclear what constitutes the actual PEPR1/2 ligands and how they are generated. Indeed, PROPEPs lack a signal peptide for secretion (14), and their secretion or processing into Pep peptides in vivo has not yet been demonstrated. Furthermore, does PROPEP production in response to elf18 also underlie the characteristic elf18-induced seedling growth inhibition? Finally, despite the emerging central role of BIK1 and related proteins in immunity, no substrates for BIK1 have been identified so far. The identification of these substrates is likely to provide invaluable insight into how the activation of PRR complexes by PAMPs or damage-associated molecular patterns leads to a multitude of responses, resulting ultimately in broad-spectrum disease resistance.
Acknowledgments
Research in the C.Z. laboratory is supported by the Gatsby Charitable Foundation, the United Kingdom Biotechnology and Biological Sciences Research Council, and the European Research Council.
Footnotes
References
- 1.Bleecker AB, Kende H. Ethylene: A gaseous signal molecule in plants. Annu Rev Cell Dev Biol. 2000;16:1–18. doi: 10.1146/annurev.cellbio.16.1.1. [DOI] [PubMed] [Google Scholar]
- 2.Pieterse CMJ, Van der Does D, Zamioudis C, Leon-Reyes A, Van Wees SCM. Hormonal modulation of plant immunity. Annu Rev Cell Dev Biol. 2012;28:489–521. doi: 10.1146/annurev-cellbio-092910-154055. [DOI] [PubMed] [Google Scholar]
- 3.Boller T, Felix G. A renaissance of elicitors: Perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Annu Rev Plant Biol. 2009;60:379–406. doi: 10.1146/annurev.arplant.57.032905.105346. [DOI] [PubMed] [Google Scholar]
- 4.Tsuda K, Sato M, Stoddard T, Glazebrook J, Katagiri F. Network properties of robust immunity in plants. PLoS Genet. 2009;5(12):e1000772. doi: 10.1371/journal.pgen.1000772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tintor N, et al. Layered pattern receptor signaling via ethylene and endogenous elicitor peptides during Arabidopsis immunity to bacterial infection. Proc Natl Acad Sci USA. 2013;110:6211–6216. doi: 10.1073/pnas.1216780110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu Z, et al. BIK1 interacts with PEPRs to mediate ethylene-induced immunity. Proc Natl Acad Sci USA. 2013;110:6205–6210. doi: 10.1073/pnas.1215543110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Monaghan J, Zipfel C. Plant pattern recognition receptor complexes at the plasma membrane. Curr Opin Plant Biol. 2012;15(4):349–357. doi: 10.1016/j.pbi.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 8.Lu D, et al. A receptor-like cytoplasmic kinase, BIK1, associates with a flagellin receptor complex to initiate plant innate immunity. Proc Natl Acad Sci USA. 2010;107(1):496–501. doi: 10.1073/pnas.0909705107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang J, et al. Receptor-like cytoplasmic kinases integrate signaling from multiple plant immune receptors and are targeted by a Pseudomonas syringae effector. Cell Host Microbe. 2010;7(4):290–301. doi: 10.1016/j.chom.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 10.Boutrot F, et al. Direct transcriptional control of the Arabidopsis immune receptor FLS2 by the ethylene-dependent transcription factors EIN3 and EIL1. Proc Natl Acad Sci USA. 2010;107(32):14502–14507. doi: 10.1073/pnas.1003347107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mersmann S, Bourdais G, Rietz S, Robatzek S. Ethylene signaling regulates accumulation of the FLS2 receptor and is required for the oxidative burst contributing to plant immunity. Plant Physiol. 2010;154(1):391–400. doi: 10.1104/pp.110.154567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu D, et al. Direct ubiquitination of pattern recognition receptor FLS2 attenuates plant innate immunity. Science. 2011;332(6036):1439–1442. doi: 10.1126/science.1204903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ji Y, Guo H. From endoplasmic reticulum (ER) to nucleus: EIN2 bridges the gap in ethylene signaling. Mol Plant. 2013;6(1):11–14. doi: 10.1093/mp/sss150. [DOI] [PubMed] [Google Scholar]
- 14.Huffaker A, Pearce G, Ryan CA. An endogenous peptide signal in Arabidopsis activates components of the innate immune response. Proc Natl Acad Sci USA. 2006;103(26):10098–10103. doi: 10.1073/pnas.0603727103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qi Z, et al. Ca2+ signaling by plant Arabidopsis thaliana Pep peptides depends on AtPepR1, a receptor with guanylyl cyclase activity, and cGMP-activated Ca2+ channels. Proc Natl Acad Sci USA. 2010;107(49):21193–21198. doi: 10.1073/pnas.1000191107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krol E, et al. Perception of the Arabidopsis danger signal peptide 1 involves the pattern recognition receptor AtPEPR1 and its close homologue AtPEPR2. J Biol Chem. 2010;285(18):13471–13479. doi: 10.1074/jbc.M109.097394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roux M, et al. The Arabidopsis leucine-rich repeat receptor-like kinases BAK1/SERK3 and BKK1/SERK4 are required for innate immunity to hemibiotrophic and biotrophic pathogens. Plant Cell. 2011;23(6):2440–2455. doi: 10.1105/tpc.111.084301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ranf S, Eschen-Lippold L, Pecher P, Lee J, Scheel D. Interplay between calcium signalling and early signalling elements during defence responses to microbe- or damage-associated molecular patterns. Plant J. 2011;68(1):100–113. doi: 10.1111/j.1365-313X.2011.04671.x. [DOI] [PubMed] [Google Scholar]
- 19.Ma Y, Walker RK, Zhao Y, Berkowitz GA. Linking ligand perception by PEPR pattern recognition receptors to cytosolic Ca2+ elevation and downstream immune signaling in plants. Proc Natl Acad Sci USA. 2012;109(48):19852–19857. doi: 10.1073/pnas.1205448109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamaguchi Y, Huffaker A, Bryan AC, Tax FE, Ryan CA. PEPR2 is a second receptor for the Pep1 and Pep2 peptides and contributes to defense responses in Arabidopsis. Plant Cell. 2010;22(2):508–522. doi: 10.1105/tpc.109.068874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schulze B, et al. Rapid heteromerization and phosphorylation of ligand-activated plant transmembrane receptors and their associated kinase BAK1. J Biol Chem. 2010;285(13):9444–9451. doi: 10.1074/jbc.M109.096842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Postel S, et al. The multifunctional leucine-rich repeat receptor kinase BAK1 is implicated in Arabidopsis development and immunity. Eur J Cell Biol. 2010;89(2–3):169–174. doi: 10.1016/j.ejcb.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 23.Laluk K, et al. Biochemical and genetic requirements for function of the immune response regulator BOTRYTIS-INDUCED KINASE1 in plant growth, ethylene signaling, and PAMP-triggered immunity in Arabidopsis. Plant Cell. 2011;23(8):2831–2849. doi: 10.1105/tpc.111.087122. [DOI] [PMC free article] [PubMed] [Google Scholar]