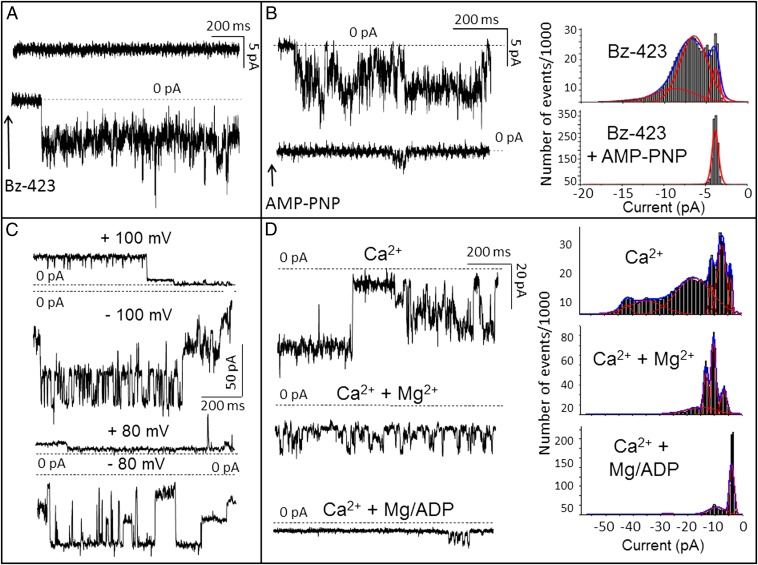
Fig. 5.
Dimers of FOF1 ATP synthase generate currents matching MMC-PTP. (A) A bilayer experiment in 50 mM KCl, 1 mM Pi, and 0.3 mM Ca2+ (Ca2+ only in trans; Vcis = −60 mV). After addition of dimeric ATP synthase, no activity could be observed (Upper) until immediately after the addition of 0.1 mM Bz-423 to the trans side (Lower; arrow). When monomers were used, the recording was identical to Upper, which was not modified by the addition of Bz-423. (B) A similar experiment in the presence of 0.1 mM PhAsO in trans. Activity (Upper) was elicited by the addition of Bz-423 as in A and inhibited by 0.1 mM AMP-PNP in trans (Lower; arrow). Corresponding current amplitude histograms from gap-free 60-s traces are shown in Right. (C) Current traces (150 mM KCl; Vcis as indicated) with dimeric ATP synthase and 0.3 mM Ca2+, 0.1 mM Bz-423, and 50 μM PhAsO added to the trans side. Note numerous substates. (D, Left) Activity (Vcis = −80 mV) recorded as in C (first trace) and after sequential additions of Mg2+ (0.6 mM) and ADP (0.6 mM) to the trans side; (D, Right) corresponding amplitude histograms from gap-free 100-s traces. Representative experiments are shown of a total of 28 experiments performed under various conditions using six different ATP synthase dimer preparations.