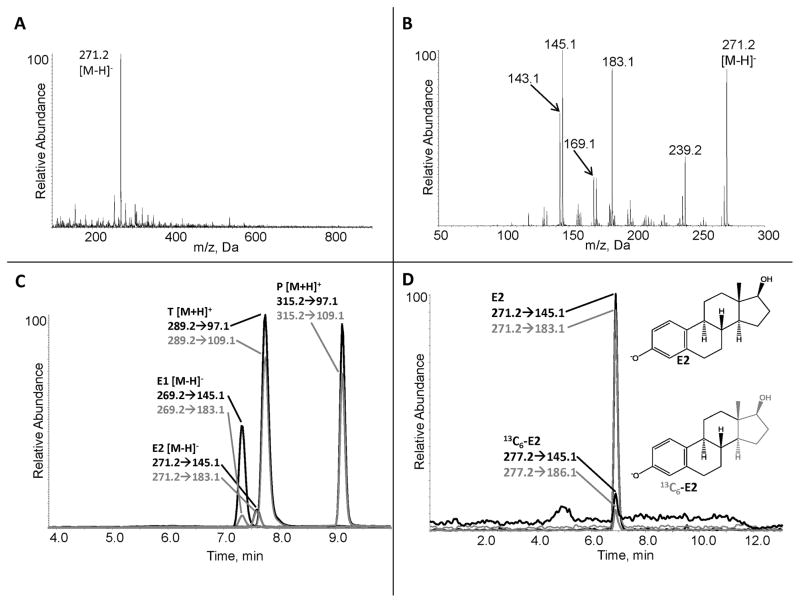
Figure 2.
Representative Data for Steroid Analysis with Mass Spectrometry A. Negative-ion MS1 spectrum of estradiol (E2) standard used to determine the ions in a sample as well as the precursor ion [M–H]−. B. Negative-ion tandem mass spectrometry (MS2) experiment used to determine the collision-induced product ions of E2, which in this case is equivalent to a full mass spectrum, since the precursor ion is the molecular ion. C. MRM ion chromatogram of estrone (E1), E2, testosterone (T) and progesterone (P). Negative (E1, E2) and positive (T, P) mass transitions were acquired during the same mass spectrometry experiment and overlaid for chromatographic comparison. To be certain a mass transition is from the steroid of interest and not contaminants, a minimum of two mass transitions (quantifier and qualifier product ions) are used; the peaks must co-elute to be validated for quantitation. The ratios of the two transitions are monitored to detect the presence of interferences. Contaminants can have an identical mass transition as the analyte, but it is very unlikely that a different compound will have two or more transitions with the same ratios as the analyte. The ratio of the two mass transitions for the endogenous analyte should be the same as the stable isotope internal standard mass transitions. D. Negative-ion MRM chromatogram of unlabeled E2 and [13C6]-E2 internal standard, which elute simultaneously. To unequivocally ensure that the mass transitions being quantitated derive from the steroid of interest and not a contaminant, it is required that the target steroid’s mass transitions co-elute with the internal standard. In order for an endogenous steroid to be quantitated, it must have two mass transitions co-eluting with the corresponding mass transitions of the isotopically labeled internal standard steroid.