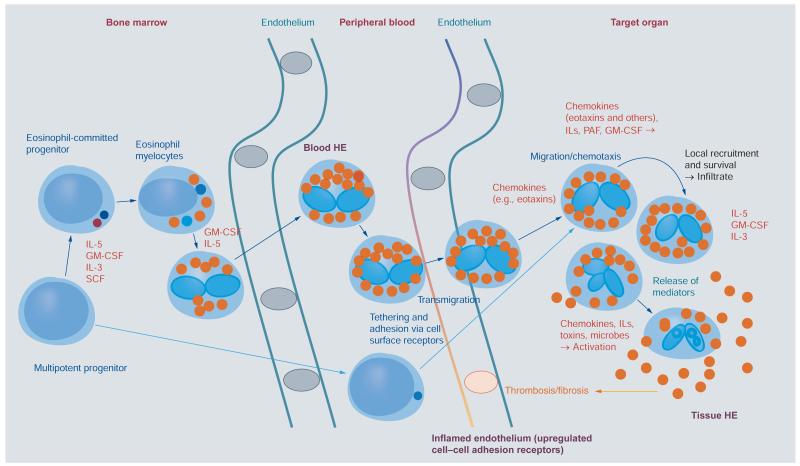
Figure 1. Development of eosinophils and reactive hypereosinophilia.
Eosinophils originate from multipotent and lineage-restricted hematopoietic progenitor cells. Eosinophil progenitors reside in the bone marrow but are also detectable in the peripheral blood. Eosinophil development is regulated by eosinophilopoietic cytokines (IL-3, GM-CSF and IL-5) and takes place primarily in the bone marrow. Cytokine-induced HE in the peripheral blood is often accompanied by tissue HE. Activation of eosinophils and activation of endothelial cells contribute to endothelial transmigration and infiltration of inflamed tissues. Eosinophil adhesion to endothelium and transmigration are mediated by certain homing receptors. Migration and accumulation of eosinophils in (inflamed) tissues are mediated by chemotactic peptides (chemokines), cytokines and other mediators. Eosinophil accumulation is also triggered by delayed eosinophil apoptosis, another cytokine-mediated phenomenon, in local tissue sites. Activation of eosinophils leads to degranulation and mediator secretion in tissues, with consequent organ damage, which may be accompanied by fibrosis and/or thrombosis and by deposition of eosinophil granule proteins.
HE: Hypereosinophilia; GM-CSF: Granulocyte/macrophage colony-stimulating factor; IL: Interleukin; PAF: Platelet-activating factor; SCF: Stem cell factor.