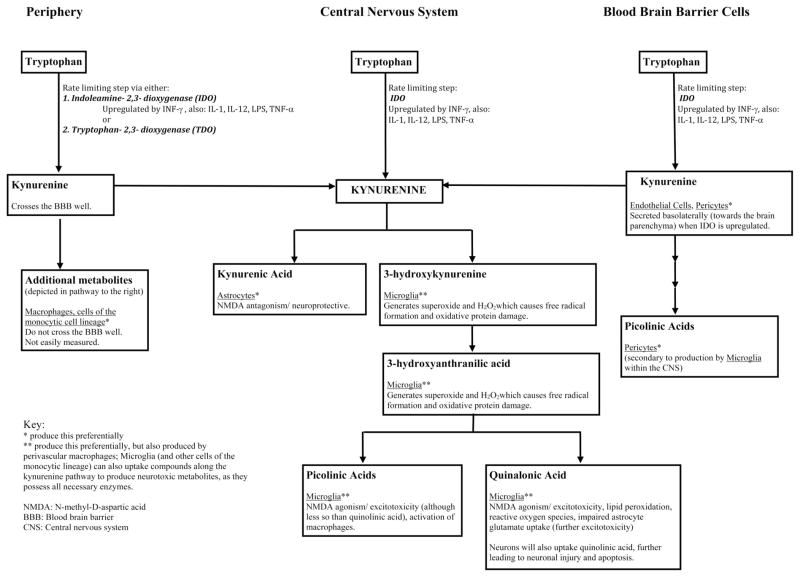
Figure 1.
Tryptophan metabolism by the kynurenine pathway. The first and rate-limiting step in the kynurenine pathway is the formation of kynurenine from tryptophan via indoleamine-2,3-dioxygenase (IDO), found ubiquitously, or tryptophan-2,3-dioxygenase (TDO). Kynurenine concentrations within the central nervous system (CNS) are related to peripheral production (42) and transportation into the brain via the large amino acid transporter in the blood brain barrier (50), basolateral secretion of kynurenine by blood brain barrier (BBB) cells (endothelial cells, pericytes) (45), or from local CNS synthesis of kynurenine pathway metabolites (i.e. astrocytes, microglia, perivascular macrophages) (45, 51). These mechanisms are upregulated by cytokines and inflammatory/immune signals, including interferon gamma (INF-γ) (48, 49), interleukins 1 and 12 (IL-1, IL-12) (63, 64), lipopolysaccharide (LPS), and tumor necrosis factor alpha (TNF-α) (65, 66), which are often increased in patients with critical illness. Increased central kynurenine concentrations lead to production of beneficial (kynurenic acid) and neurotoxic kynurenine pathway metabolites. At times of stress and with inflammation, the pathway preferentially produces neurotoxic metabolites including quinolinic acid, 3-hydroxy-kynurenine, anthranilic acid, and picolinic acids (41, 42, 45, 51). An imbalance favoring the production of neurotoxic metabolites leads to neuronal and glial cell injury, excitotoxicity, and apoptosis (30, 32, 37, 43–47), which may be clinically manifested as delirium or coma.