Abstract
Aging is a complicated process characterized by a progressive loss of homeostasis, which results in an increased vulnerability to multiple diseases. HIV-1-infected patients demonstrate a premature aging phenotype and develop certain age-related diseases earlier in their lifespan than what is seen in the general population. Age-related comorbidities may include the development of bone disease, metabolic disorders, neurologic impairment and immunosenescence. Age also appears to have an effect on traditional markers of HIV-1 disease progression, including CD4+ T-cell count and viral load. These effects are not only a consequence of HIV-1 infection, but in many cases, are also linked to antiretroviral therapy. This review summarizes the complex interplay between HIV-1 infection and aging, and the impact that aging has on markers of HIV-1 disease.
Keywords: aging, comorbidities, disease progression, HIV-1, neurocognitive impairment
Aging is a complicated biological process involving numerous intricately linked intrinsic and extrinsic factors affecting different systems of the human body. Several unique medical comorbid factors are observed with increasing frequency with aging or senescence. In addition to the increased vulnerability to disease associated with advancing age, typical changes related to aging include changes in hearing, vision, bone strength and density and immune function. Because life expectancies are increasing, particularly in the developed world, the incidence of aging-related disease is expected to increase dramatically in the coming decades.
In a subset of the aging population in the developed world, treatment of individuals infected with HIV-1 has improved significantly since the introduction of combination antiretroviral therapy (ART), also known as HAART. Mortality and morbidity have decreased significantly and life expectancy has increased dramatically in this subpopulation as a result of HAART [1-4]. As a result, HIV-1 has become an epidemic that is increasingly affecting older adults. By 2015, it is estimated that approximately half of patients infected with HIV in the USA will be over the age of 50 years, making it paramount to understand the risk factors associated with aging and HIV-1 infection [5].
Although HAART has greatly improved mortality rates in infected patients when compared with no treatment or monotherapy, a large discrepancy in life expectancy persists between HIV-infected individuals and the general (noninfected) population. Studies have shown that a 20-year-old infected individual on HAART with a nadir CD4 count of between 100 and 200 cells/μl can expect to live approximately 42 years, which is only about two-thirds as long as the general population. This number increases to 50 years in those with a nadir CD4 count of greater than 200 cells/μl and falls to 32 years in those with a nadir CD4 count of less than 100 cells/μl [6-8]. Interestingly, despite these discrepancies, mortality resulting from AIDS-related illnesses has decreased dramatically. HIV-1-infected individuals are also at increased risk of non-AIDS-related illnesses traditionally associated with aging, including cardiovascular disease and neurocognitive decline. This has led to the concern that HIV-1-infected individuals may suffer from accelerated aging and neurocognitive impairment or comorbidities normally associated with advanced age much sooner than the general population.
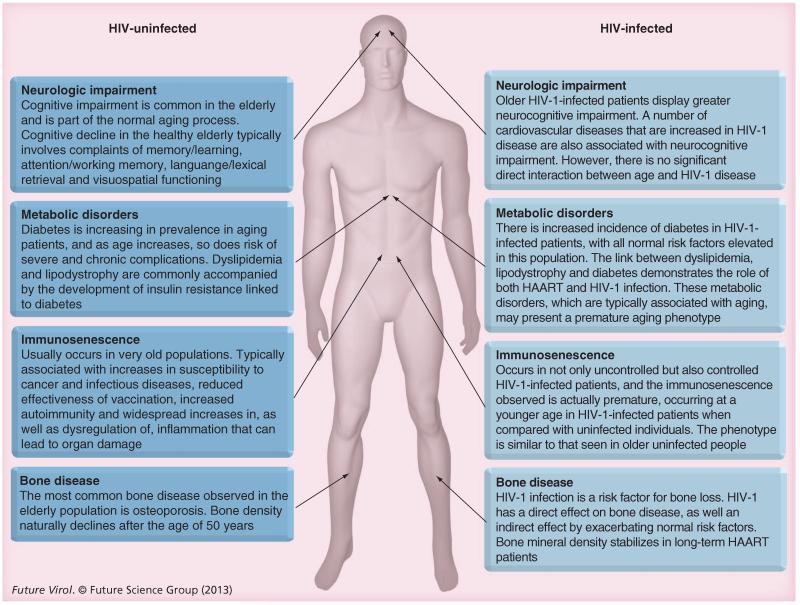
Aging-related comorbidities within the HIV-1-infected population
Aging is a complicated process involving several intrinsic and extrinsic factors intricately linked together, involving different systems of the human body. Common aging-related chronic conditions include diabetes, lipidemia, cardiovascular disease, immune dysregulation, changes in bone strength and density and neurologic impairment, including dementia syndromes such as Alzheimer’s disease (AD) and vascular dementia. These diseases are progressive and are often treatable, but not curable, and often coexist within the aging population [9-11]. Prevalence of these diseases is increased among HIV-1-infected patients [12,13] and current research suggests that this may be the result of premature aging associated with HIV-1 infection [14,15]. Furthermore, the simultaneous presence of two or more of these aging-related diseases is more common among HIV-1-infected patients than in the general population when looking at a wide spectrum of ages [16]. Interestingly, the prevalence of multiple aging-related diseases among HIV-1 -infected patients is equivalent to prevalence among members of the general population who are 10–15 years older [17-19]. This supports the notion that accelerated/premature aging in the HIV-1-infected population contributes to the increase in aging-related diseases.
Diabetes, dyslipidemia & lipodystrophy
Diabetes is a serious metabolic condition that is increasing in prevalence in the elderly. By 2025, adults aged 60 years and older are expected to comprise approximately two-thirds of the diabetic population in developed countries [20]. Minority populations may be particularly affected [20]. With increased age comes the risk of more severe and chronic complications associated with diabetes [21]. In addition to a rise in the incidence of diabetes in the general aging population, the decreased mortality associated with controlled HIV-1 infection has led the incidence of diabetes to increase in this population. Some research has suggested that HIV-1 infection can cause islet B-cell dysfunction, leading to insulin-dependent diabetes in the absence of islet cell or insulin antibodies [22]. Although HIV-1 infection alone has not been strongly associated with an increased risk of diabetes, increasing age within this population has been associated with increased risk [23]. Increasing age, male sex, minority race and elevated BMI are all associated with increased risk of diabetes in the general population. The effect of these factors is more pronounced in the HIV-1-infected population; thus, their risk of diabetes is higher [23].
While a number of clinical factors have been associated with increased risk of diabetes in both the HIV-1-infected as well as the elderly population, an even greater association occurs between diabetes and the use of HAART (Figure 1). Long-term HAART treatment, which is largely responsible for the decreased mortality associated with HIV-1 disease, is also associated with an increased risk of diabetes. Early in the HIV-1 epidemic, when no treatment options were available and many patients rapidly developed AIDS, toxicity and efficacy assessments of new antiretroviral compounds were limited to identifying the most immediate problems and major concerns associated with these agents. Some abnormalities, including metabolic diseases, were considered to be secondary to the control of the severe immune deficiency affecting these patients [24,25]. However, as therapy regimens improved and the lifespan of HIV-1-infected patients increased, addressing the secondary toxicities and problems associated with these treatments has become increasingly important. Studies have now directly demonstrated that some antiretroviral compounds are associated with an induction of insulin resistance and onset of diabetes [26-28]. Risk of diabetes onset increases with the use of nucleoside reverse transcriptase inhibitors (NRTIs) and to a lesser extent with non-NRTIs [23,27]. Mitochondrial toxicity associated with the use of NRTIs likely plays a role; mitochondrial DNA (mtDNA) content is decreased with the use of compounds such as stavudine, zidovudine and didanosine [26,27,29,30]. However, the role of protease inhibitors (PIs) cannot be overlooked; PIs are rarely given alone and may play a role in the risk associated with NRTIs [23,27]. Indinavir and ritonavir in particular appear to interfere with insulin-stimulated glucose uptake, resulting in insulin resistance [31]. A cumulative dose effect from the actions of multiple compounds may explain the increased risk of diabetes onset.
Figure 1. HIV-1 infection initiates a premature aging response.
Normal aging-related disease states that are observed in the elderly occur at much younger ages in HIV-1-infected patients. These disease states include enhanced neurologic decline, metabolic/cardiovascular diseases including dyslipidemia, lipodystrophy and diabetes, immune system dysfunction and bone disease. HAART also seems to play a role in premature aging.
Although evidence suggests a direct connection between diabetes and HAART, there may also be an indirect mechanism involving lipoatrophy. HAART has been associated with fat redistribution syndrome (lipodystrophy) that may include both central fat accumulation (lipohypertrophy) and peripheral fat wasting (lipoatrophy) [32]. Changes in body fat redistribution are commonly accompanied by the development of insulin resistance [33-37]. In lipoatrophy, increased lipolysis reflects insulin resistance within adipose tissue. Lipolysis results in increased circulating free fatty acids, which reinforces insulin resistance in both the liver and skeletal muscles [35-37]. Visceral fat accumulation as seen in lipohypertrophy is also associated with an increase in cardiometabolic risk factors, including increases in triglyceride levels and a decrease in insulin sensitivity [38-43]. This holds true in both the HIV-1-infected population and the general population. Patients on HAART treatment that includes stavudine and/or didanosine have increased lipoatrophy compared with patients on regimens that do not include these agents, suggesting a connection between therapy-induced lipoatrophy and insulin resistance [26,28,35-37,44,45]. The connection between lipoatrophy and didanosine is more controversial, with some studies demonstrating no link [46]. A more severe metabolic syndrome includes lipodystrophy, severe dyslipidemia, and insulin resistance. This disorder – HAART-associated dyslipidemic lipodystrophy – is likely caused by antiviral therapy, which can include NRTIs and PIs. Dyslipidemia itself is characterized by abnormal lipid and lipoprotein profiles and occurs in a high proportion of HIV-1-infected patients, particularly those treated with PIs or certain NRTIs, including stavudine, zidovudine, didanosine, lamivudine and abacavir [18]. Dyslipidemia patients typically present with elevated serum triglycerides and total cholesterol levels, decreases in high-density lipoprotein (HDL) cholesterol and increases in low-density lipoprotein (LDL) and very low-density lipoprotein cholesterol levels. Prior to HAART, it was shown that HIV-1 caused dyslipidemia with declines in total cholesterol levels [47-49]. Untreated patients infected with HIV-1 have been shown to have not only low total cholesterol, but also lower levels of LDL and HDL in conjunction with elevated serum triglyceride levels [25,50,51]. Lower HDL levels have been connected to higher levels of HIV-1 RNA circulating in the periphery [52,53]. Following the introduction of HAART, it has been demonstrated that therapy also plays a role in dyslipidemia. It has been hypothesized that the catalytic region that PIs target in the HIV-1 protease is homologous with two human proteins that regulate lipid metabolism. The homologies with these two proteins, CRABP-1 and LDL receptor-related protein, and the potential targeting of these two proteins by PIs may be the cause of the dyslipidemia and lipodystrophy with the associated insulin resistance in PI-treated patients [32,54-56].
The link between dyslipidemia, lipodystrophy and diabetes suggests that treatment regimens as well as HIV-1 infection itself may play a role in causing metabolic complications. The metabolic complications in patients not infected with HIV-1 are typically observed in an aging population, and are generally responsible for increased cardiovascular and hepatic disease. This suggests that these metabolic disorders, which are typically associated with aging, may present a premature aging phenotype, but at the same time may promote and participate in premature aging by linking to other aging-related diseases (Figure 1).
Immune dysregulation (immunosenescence)
Immunosenescence typically occurs in individuals greater than 70 years old. It is a general term used to describe an age-related decline in immune competence marked by alterations in the overall function of the immune system [57]. Immunosenescence is characterized by an increase in the number of terminally differentiated effector memory CD8+ T cells that are generally characterized by the inability to proliferate, the absence of CD28 expression, shortened telomeres, loss of telomerase activity and enhanced secretion of inflammatory cytokines [58]. The number of naive CD8+ T cells also tends to decrease. In addition, the CD4+:CD8+ T-cell ratio decreases, overall T-cell activation increases, T-cell proliferation and thymic involution are reduced and levels of many inflammatory mediators increase. Typically, immunosenescence in the elderly is associated with increases in susceptibility to cancer and infectious diseases, reduced effectiveness of vaccination, increased autoimmunity and widespread increases in, as well as dysregulation of, inflammation, which can lead to organ damage [58-63]. Immunosenescent phenotypes are generally accelerated by the presence of chronic infections, with CMV implicated most often in aging. The term ‘immune risk phenotype’ was coined to describe the combination of a high CD8+ T-cell count and low CD4+ T-cell count with poor proliferative response to concanavalin A as well as low percentages of B cells [64,65]. There was a 2–4-year decrease in survival rate observed in patients aged 85 years and older in Sweden involved in part of the OCTO study [66]. The definition was later revised to only include a CD4+:CD8+ T-cell ratio and then revised again to include persistent CMV infection. The decrease in survival rate among patients exhibiting the immune risk phenotype was also observed in the NONA study, with included elderly patients not necessarily selected for good health [67].
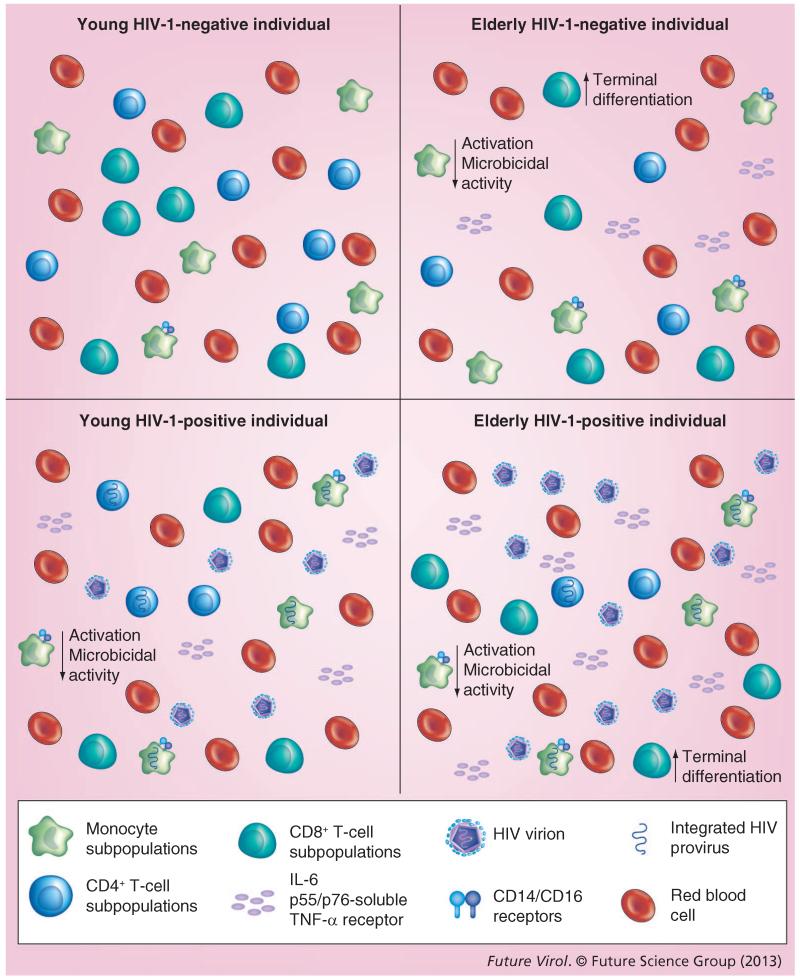
With HAART resulting in a chronic type of HIV-1 infection, in which patients with well-controlled disease potentially live for several decades, immunosenescence is occurring in patients with controlled disease as well as in those with uncontrolled disease. The immunosenescence observed appears to be premature, occurring in younger HIV-1-infected patients compared with the general population (Figure 1). The phenotype is similar to that observed in older, noninfected people (Figure 2). However, new evidence suggests that premature aging may occur not only in the CD8+ T-cell population, but also in specific subsets of naive CD4+ T cells [68]. Patients who do not regain a normal CD4+ T-cell population during therapy are more likely to demonstrate the immunosenescence phenotype, compared with those in whom CD4+ T cells are restored [69-71]. Good thymic output of naive T cells correlates with a good immunological response to treatment. Studies suggest that premature aging phenotypes in both the CD8+ and CD4+ T-cell compartments predict faster clinical disease progression [72]. HIV-1-associated immunosenescence may thus contribute to long-term continued immunodeficiency as well as to premature aging-associated diseases in infected patients [19,73]. Investigators are exploring ways to increase immune response to therapy, boost the naive T-cell production and either restore function to senescent immune cell populations or deplete these dysfunctional cells.
Figure 2. Immune regulation in response to aging and HIV-1 infection.
As an individual ages, immune competence declines and changes occur in the overall function of the immune system. With aging, the number of terminally differentiated CD8+ T cells increases, while their ability to proliferate decreases and CD28 expression decreases. There are also fewer naive CD8+ T cells. Aging also decreases the number and function of CD4+ T cells. This decrease has also been shown to lead to a decrease in the overall CD4+:CD8+ T-cell ratio. HIV-1 infection itself also induces changes in the overall number and function of CD4+ T cells, increasing the number of terminally differentiated CD8+ T cells and decreasing the ability of CD8+ T cells to proliferate. Even though the number of CD4+ T cells decreases, the number of memory CD4+ T cells increases; however, these memory cells are less able to respond to pathogens. Blood monocytes and tissue macrophages typically have a decreased level of activation in older, uninfected patients. Large increases have also been shown in CD14+/CD16+ receptors on mature monocytes in the elderly. This was similar to what was observed in younger HIV-1-infected patients. Within both the older uninfected population, as well as HIV-1-infected patients, macrophages appeared to have diminished microbicidal capability. However, not all aging-related changes have centered on a loss of function. IL-6 cytokine levels increase with aging, as well as independently with HIV-1 infection. p55- and p75-soluble TNF-α receptor levels also increase. These levels all increase further in HIV-1-infected patients as they age.
Bone disease
The most common bone disease observed in the elderly is osteoporosis. Osteoporosis is classically defined as an imbalance between bone resorption and formation. Osteoporosis can develop in several ways, including excessive bone resorption, inadequate levels of new bone formation during remodeling or an interplay between these mechanisms [74]. Generally, bone regeneration is marked by bone resorption by osteoclasts and new bone formation occurring via osteoblasts. Peak bone mass usually occurs at approximately 30 years of age, after which bone density naturally declines because the rate of osteoclast resorption outpaces new bone formation by osteoblasts. The WHO classifies general bone health into three categories: normal, osteopenia and osteoporosis [75]. Dual-energy x-ray absorptiometry is used to assess bone health by measuring bone mineral density (BMD). In addition to age, factors increasing the risk of bone loss include female sex, race, lifestyle, diet, body size, menopause and hormone treatment. Osteoporotic bone is less dense, weaker and much more prone to fractures and breaks [76,77].
HIV-1 infection is also a risk factor for bone loss or disease (Figure 1). Low BMD has been reported in studies involving both older [78-81] and younger [77,82-86] HIV-1-infected patients. Bone fracture rate is 30–70% higher in HIV-1-infected patients compared with uninfected controls [87-89]. The mechanism behind low BMD in HIV-1-infected patients is complex. Chronic HIV-1 infection appears to exacerbate osteoporosis risk factors, such as poor nutrition and weight loss. HIV-1-infected patients are also more likely to use tobacco and alcohol, which increase osteoporosis risk [85,90-92]. HIV-1 infection itself also appears to directly affect bone loss. HIV-1 proteins increase osteoclastic activity and promote osteoblast apoptosis [93]. Increases in TNF-α, an inflammatory cytokine commonly upregulated in HIV-1 infection, have also been shown to result in increased bone resorption by osteoclasts [94]. Studies have also shown that uncontrolled viremia may impact BMD; therapy-naive HIV-infected patients have a high prevalence of osteopenia [77,95]. The initiation of HAART may also induce a significant loss in BMD, regardless of the compounds used [92,96,97]; agents implicated include tenofovir– and atazanavir–ritonavir [92,98-102]. BMD appears to stabilize in patients receiving long-term, established HAART [90,91,103,104]. Vitamin D is important for cell growth, immunity and metabolism [105]. Vitamin D deficiency has been associated with decreased BMD and increased risk and severity of bone diseases. Current research also suggests that vitamin D deficiency may be linked to an increased risk of HIV-1 infection and enhanced disease progression. Research has suggested that vitamin D supplementation along with HAART may improve bone health in infected patients while simultaneously controlling HIV-1 replication, increasing CD4+ T-cell counts, slowing the rate of HIV-1 disease progression, decreasing the risk of HIV-1-related neurocognitive decline and improving overall survival [105,106]. However, much more research is needed to fully understand the effects of vitamin D on infected individuals, the mechanisms involved and the concentrations to be used.
Risk factors for increases in bone loss in HIV-1-infected patients, including those on HAART, affect all age ranges. The prevalence of fractures in HIV-1-infected patients is 62% higher than in uninfected patients, again spanning all age ranges [87]. As the HAART-treated population grows older, bone disease may increase not only from disease, but also with age [12]. Recent studies have demonstrated significantly decreased BMD in postmenopausal HIV-1-infected women, as well as a higher level of bone turnover markers, further increasing the risk of fractures and breaks for these women [107]. Little is known about when to screen HIV-1-infected patients at risk for bone loss, and few studies have assessed the efficacy of common treatments for bone loss in HIV-1-infected patients. Thus, studies assessing appropriate diagnosis and treatment options for bone loss in this growing population deserve strong emphasis.
Neurologic impairment
Cognitive decline without dementia is common among older patients and is thought to be a component of normal aging (Table 1) [108]. Cognitive decline in the healthy elderly population typically involves problems with memory/learning, attention/working memory, language/lexical retrieval and visuospatial functioning. Decline in cognitive functioning may impair an older person’s ability to perform instrumental activities of daily living, such as managing personal finances [108,109]. Underlying disease states unrelated to aging, such as HIV-1 infection, can also cause cognitive decline.
Table 1. Neurologic complications associated with aging and HIV-1 infection.
| Observation | Issues | Ref. |
|---|---|---|
| Older HIV+ patients display greater neurocognitive impairment |
Utilized large cohorts of HIV+ and HIV− patients, but uninfected control group was younger than HIV+ group |
[116] |
| Age effects seen in older HIV+ patients as compared with younger HIV+ patients |
Lacked young and old HIV− control groups |
[127] |
| Differential decline in cognitive impairment with increasing age in HIV+ patients |
Sample of older HIV− patients was small |
[128] |
| No significant interaction between age and neurocognitive impairment |
None. Well-controlled study using a well-established cohort |
[129] |
| Decreased concentrations of N-acetylaspartate in frontal white matter suggest mitochondrial toxicity |
Only looked at four antiretroviral compounds |
[130] |
| Cardiovascular diseases, including dyslipidemia, hypertension and diabetes, associated with neurocognitive impairment in HIV+ patients, and greater neurocognitive score |
[114,115,132] | |
| Diffusion-weighted MRI found an association between abnormal glucose metabolism and lower fatty acid in caudate nucleus and hippocampus |
Effects were mitigated with age and education adjustment |
[134] |
HIV-associated neurocognitive disorder refers to a wide variety of neurologic disorders from mild cognitive impairment to HIV-associated dementia (Figure 1) [110,111]. Drug treatment has substantially reduced HIV-associated dementia, but HIV-1-related neurocognitive disorders are an increasing problem in patients aged 50 years and older [112,113]. Results suggest that HIV-1-related neurocognitive disorders may be potentiated by common age-associated medical problems, including hypertension [114], hypercholesterolemia [115] and diabetes [116]. Evidence also suggests that HIV-1-related neurocognitive impairment occurs in conjunction with biomarkers commonly seen in patients with AD, with or without vascular comorbid factors such as β amyloid and total τ [117,118]. An abundance of research shows the presence of neuropathological markers commonly seen in AD in individuals with HIV-1-related neurocognitive disorders [119]. In these studies, HIV-1-infected participants had decreased β amyloid and increased total τ in cerebrospinal fluid (CSF). In one study, CSF samples were examined from three groups: HIV-1-infected patients with intact or impaired cognition and normal, uninfected control subjects [120]. HIV-1-infected patients with neurocognitive impairment had lower CSF β amyloid levels, comparable to levels in patients with mild AD. Total τ was higher for AD patients, but lower than levels in HIV-1-infected subjects or normal, uninfected participants. Well-established dementia-related factors such as the ApoE ε4 genotype also appear to contribute to HIV-1-related neurocognitive disorders. Thus, a variety of age-related cardiovascular risks may contribute to HIV-1 neurocognitive disorders. According to one model, age-related medical problems occurring in conjunction with HIV-1-related biologic substrates act synergistically and result in a higher incidence, or early emergence, of HIV-1-related neurocognitive disorders [121-124].
Recent research underscores a variety of important clinical and methodological factors in evaluating the origins and clinical course of neurocognitive problems in older patients with HIV-1 infection [125]. First, older patients typically present with longer disease duration and longer length of treatment with antiretroviral medications [112,113]. Also, some of these patients survived the period when appropriate pharmacological treatment was not yet available, and some were treated with therapies more toxic than compounds currently available. Their longer survival, coupled with the effects of HAART, poses a challenge for prospective research assessing the unique contribution of age to HIV-1-related neurocognitive deficits.
Several studies have examined age-related neurocognitive impairment in HIV infection (Table 1). Becker et al. described greater neuropsychological impairment in older compared with younger patients in a large cohort of HIV-1-infected patients, compared with a control (uninfected) population [116]. Twenty-three percent of older HIV-infected patients versus 9% of younger patients had evidence of dementia. The control group in this study, however, was younger than the HIV-1-infected cohort. Studies have also described age effects as they relate to serostatus; neurocognitive test results were more variable among older than younger HIV-1-infected patients, and among older study participants not infected with HIV-1 [126]. An additional study reported age effects among older HIV-1-infected patients; however, this study lacked a comparable study group not infected with HIV-1 [127]. Another study showed worse cognitive impairment in older HIV-1-infected patients; however, in this study, the sample of older patients not infected with HIV-1 was quite small [128]. Finally, a study examining patients from the Hawaii Aging with HIV Cohort found no striking interaction between age and neurocognitive status [129]. This study carefully controlled for potentially confounding demographic variables. All of these studies illustrate important methodological factors in studying the relationships among age, HIV infection and neurocognitive function. They suggest that age might best be used as a continuous rather than a categorical variable and raise the question of whether age should be treated as a linear or nonlinear variable.
ART may also have neurocognitive effects. One study attempting to define the effects caused by therapy examined patients taking stavudine or didanosine, both of which are linked to mitochondrial toxicity, versus zidovudine or lamivudine, which are thought to be less toxic. These studies found decreased concentrations of brain N-acetylaspartate in patients taking stavudine or didanosine in frontal white matter, a common marker of HIV-1-associated neurocognitive impairment [130]. This finding would be compatible with compromised mitochondrial integrity. A second issue involves whether newly evolved neurocognitive impairment in older HIV-1 patients was accompanied by prior monotherapy versus dual therapy. The impact of these treatment regimens in older patients with emerging neurocognitive deficits requires further investigation. Third, the issue of cognitive reserve should be considered. In the literature on dementia, cognitive reserve is viewed as a mechanism that either delays onset of cognitive disabilities or limits the severity of cognitive disabilities over illness duration [131]. The impact of cognitive reserve in older patients with HIV-1 disease has not been examined.
Studies have suggested that factors that increase risk for dementia, including smoking, dyslipidemia, hypertension and diabetes mellitus, are increased in the HIV-1-infected population and can be associated with greater cognitive impairment in these patients [114,132]. Comorbid cardiovascular diseases, including hypertension and hypercholesterolemia, are associated with impaired performance on neurocognitive testing [115]. In a large cohort of HIV-1-infected patients, subclinical atherosclerosis, measured by carotid intima–media thickness (a proxy for cerebrovascular compromise), was related to slower performance on tests assessing psychomotor operations [133]. In another study, researchers employed diffusion tensor imaging to study brain alterations in HIV-infected individuals with or without cerebrovascular risk factors [134]. Diffusion-weighted MRIs assessing fractional anisotropy and mean diffusivity were obtained. Abnormal glucose metabolism was associated with lower fractional anisotropy in the caudate nucleus and hippocampus. However, effects were mitigated when scores were adjusted for age and education. It is likely that HIV-1 infection conveys risk to the integrity of subcortical nuclei. However, understanding the specific effects of illness related to cerebrovascular disease will require more detailed studies.
Effects of aging on markers of HIV-1 disease progression
Numerous factors linked to progression of HIV infection (Table 2) have been previously reviewed [135]. These factors include immunologic, virologic, host and viral genetic and host-specific factors, such as age, sex and mode of transmission. Understanding these factors and defining the parameters that affect disease progression will facilitate the development of new therapeutic agents and improve treatment decision-making. These ‘markers’ include classical factors such as CD4+ T-cell count and HIV-1 viral load (VL) measurements. Age itself is an important predictor of HIV-1 disease progression. HIV infection progresses to AIDS more rapidly in older than in younger patients, and mortality is much higher in older patients who develop an AIDS-defining illness [136].
Table 2. Markers of HIV-1 disease progression are affected by age.
| Marker | Young HIV-1- negative individual |
Elderly HIV-1-negative individual |
Young HIV-1-positive individual |
Elderly HIV-1-positive individual |
|---|---|---|---|---|
| Viral load | Not applicable | Not applicable | Efficacy of and response to therapy is strongly linked to baseline viral levels [140,141] |
Typically present with higher viral loads at time of diagnosis [142-144] Some studies show no difference between younger and older patients with regard to response to HAART [144-155], while others show increased response to therapy in older patients [149,156-164] |
| CD4 count | Normal CD4+ T-cell counts and cellular function |
Age induces changes in the number, proportion and function of CD4+ T cells [176] |
HIV-1 infection is associated with decreased capacity for replication of T-cell precursor cells [182] Respond well to HAART with CD4 T-cell rebound [183-186] |
Older patients present with lower CD4+ T-cell counts at the time of diagnosis [187,188] Some studies show a decrease in response to HAART in older patients [70,147-149,153,154,156,157,189-195], while others show no difference [145,150-152,155,159-161,164,196,197] |
| Monocytes and macrophages |
Normal cell counts and proper functioning of blood monocytes and tissue macrophages |
Decreased levels of activation in older patients, with decrease in MHC class II HLA-DR [198,199,207] Increase in the minor CD14+CD16+ cell population [199,200] Decreased microbicidal activity [208-210] Increase in basal levels of IL-6 and p55-soluble TNF-α receptor, as well as p75- soluble TNF-α receptor [213,215,216] |
Increase in the minor CD14+CD16+ cell population [199,200-202] Decreased microbicidal activity [208-212] Increase in basal levels of IL-6 and p55-soluble TNF-α receptor, as well as p75- soluble TNF-α receptor [213,215,216] |
Decreased levels of activation in older patients, with decreases in MHC II HLA-DR [198-200] Increase in the minor CD14+CD16+ cell population [199,200-202] Decreased microbicidal activity [208-212] Increase in basal levels of IL-6 and p55-soluble TNF-α receptor, as well as p75-soluble TNF-α receptor [213,234,235] |
| mtDNA | Maintains normal functioning and does not yet begin to accumulate the large deletions associated with older age |
Large deletions accumulate in a variety of tissues [230] Direct correlation between the amount of mtDNA lesions [231] Mice bearing a defective allele of the mtDNA polymerase-γ exhibit a premature aging phenotype [232] |
HAART has been shown to directly inhibit DNA polymerase-γ [233,234] Patients harboring a polymorphism at E1143 of the polymerase-γ gene have a fourfold higher risk for the development of lipodystrophy while on HAART [236] Haplogroups of mtDNA were associated with differences in metabolic response to antiretroviral therapy in an independent study [237], although the development of lipoatrophy was not related to mitochondrial haplogroups in a separate study [238] |
HAART therapy has been shown to directly inhibit DNA polymerase-γ [234,235] Patients harboring a polymorphism at E1143 of the polymerase-γ gene have a fourfold higher risk for the development of lipodystrophy while on HAART [236] Haplogroups of mtDNA were associated with differences in metabolic response to antiretroviral therapy in an independent study [237], although the development of lipoatrophy was not related to mitochondrial haplogroups in a separate study [238] |
mtDNA: Mitochondrial DNA.
Viral load
VL measurements (HIV-1 viral RNA quantification from peripheral blood) have long been used as a prognostic marker of HIV-1 disease progression [137-139]. In many patients, VL and CD4+ T-cell count are inversely related, as are VL and survival time [140,141]. Disease progression is considerably increased in patients with HIV-1 RNA levels >100,000 copies/ml, regardless of CD4+ T-cell count. Extensive research has demonstrated that, unlike CD4+ T-cell counts, HIV-1 RNA levels from later time points may be better indicators of disease progression, with a stable viral set-point not being reached until after 1 year of infection. Treatment efficacy and response to therapy are strongly linked to baseline VL levels. Levels greater than 150,000 copies/ml correspond to a 1.5-fold increased likelihood of treatment failure (meaning the ability to decrease the VL to <50 copies/ml).
Studies have shown that older patients present with higher VL measurements at diagnosis (Table 2) [142-144]. Regarding virological response to HAART treatment, many studies have found no difference between younger and older patients [145-155], whereas others have found a better response in older patients [149,156-164]. The better virologic response observed in older patients may reflect better adherence to HAART [154,161,165,166], but this has not always been the case [156].
CD4+ T-cell counts
CD4+ T cells are important mediators of the specific immune response to infection and are a primary target of HIV-1 infection. As infection progresses, the number of CD4+ T cells declines, restricting the ability of the immune system to respond to other invading pathogens (Figure 2) [167]. To date, the CD4+ T-cell count is the most significant and the most utilized predictor of disease progression [168-174], and the overall treatment protocol is usually initiated in response to a decline in CD4+ T-cell count [137]. In the past, in resource-rich settings, a CD4+ T-cell count of approximately 350 cells/μl was the treatment threshold, whereas in resource-limited settings, 200 cells/μl was the treatment threshold. This lower threshold is thought to double the risk for disease progression [170]. However, current thinking suggests that treatment should either be initiated when the CD4+ T-cell count reaches 500 cell/μl, or that treatment should be given to every infected individual, regardless of CD4+ T-cell count. In addition to determining when to start HAART treatment, CD4+ T-cell count has been used to monitor the efficacy of treatment regimens [137,175].
Both age and HIV-1 infection have been shown to independently induce changes in the number and function of CD4+ T cells (Figure 2 & Table 2). In elderly HIV-negative patients, immunosenescence leads to a decrease in CD4+ T-cell counts [15,176-178]. Thymic involution, which occurs with aging, results in a decreased ability to replace CD4+ T cells that are depleted by HIV-1 infection [179-181]. In addition to the decreased ability to replace CD4+ T cells, HIV-1 infection is associated with a decreased capacity for the growth of T-lymphocyte precursor cells [182]. Although the number of memory CD4+ T cells increases with age, their ability to respond to primary pathogens decreases [183-186]. Studies have shown that older patients present with lower CD4+ T-cell counts at diagnosis [187,188]. Thus, immunologic recovery may be less robust in older HIV-positive patients than in younger patients. A recent study involving 1956 patients showed an inverse relationship between CD4+ T-cell recovery and patient age on multivariate analysis [189]. Although many studies concur with these results and suggest a decline in CD4+ T-cell count and in CD4+ T-cell response to HAART treatment with increasing age [70,147-149,153,154,156,157,189-195], controversy continues regarding this observation. A recent study examining 101 elderly HIV-1-infected patients and 202 matched, younger HIV-1-infected patients showed a similar rate of response in the two groups with respect to CD4+ T-cell increases following the initiation of HAART [152]. Other studies support this finding [145,150-152,155,159-161,164,196,197]. However, greater compliance among older patients may explain these results [154,161,165,166].
Monocyte-macrophage populations
In comparison to the adaptive immune response, less is known about the effects of age on the innate immune response. The proper functioning of blood monocytes and tissue-resident macrophages is paramount in controlling inflammation, and these cell populations play a large role in the development of age-related inflammatory conditions [198]. Studies have demonstrated a large increase in the minor population of CD14+CD16+ monocytes displaying a mature phenotype in older patients [199,200]. This cell population has been shown to be increased in untreated HIV-1-infected individuals [201,202] and is thought to play a major role in trafficking HIV to the brain [203-206]. Macrophages in older patients have decreased levels of activation, as demonstrated by a decrease in the expression of MHC class II HLA-DR activation molecules (Figure 2) [199,207]. Activation of macrophages is key for the proper functioning of these cells. The microbicidal capacity of macrophages also appears to diminish with age. This is reflected in weakened respiratory bursts, decreases in reactive nitrogen and oxygen intermediates and decreased capacity to complete phagocytosis [208-210]. Similar changes in function occur in response to HIV-1 infection. This functional change has been shown to be mediated by Nef and is related to the decrease in CD4+ T cells (Table 2)[211,212].
However, not all age-related effects on monocytes and macrophages have been associated with a decrease in function (Figure 2). Older patients have elevated basal plasma levels of IL-6, a proinflammatory cytokine that can be secreted by both monocytes and macrophages. Increases in IL-6 have been considered a risk marker for the development of atherosclerosis [213]. Elevated levels of IL-6 are also detected in younger HIV-1-infected patients compared with healthy control patients [214]. Other proinflammatory responses observed in older patients include increases in high-affinity p55- and p75-soluble TNF-α receptor levels [215,216]. These receptors have also been shown to be elevated in HIV-1-infected patients and are linked to both immune activation and disease progression [217,218]. These factors remain elevated even after 6 years of undetectable VL [219]. These studies suggest that the phenotype of innate immune functioning observed in older patients matches the phenotype detected in response to HIV-1 infection and may play a role in the accelerated aging effects reported in younger HIV-1-infected patients.
Mitochondrial effects
Aerobic respiration in eukaryotes occurs in the mitochondria, which are involved in the primary energy-yielding reactions within the cell. Mitochondria produce 92% of cellular ATP and play critical roles in calcium regulation, thermogenesis and apoptosis [220]. Within the cell, mitochondria exist as a highly dynamic network that is actively remodeled through fission and fusion events [221]. The mitochondrial genome has been shown to reside within the matrix. Unlike the multichromosome configuration of nuclear DNA, which contains both introns and exons, the human mitochondrial genome is intronless, circular and approximately 16,569 base pairs in size [222]. Depending on cell type, circular mtDNA copies can range from 1000 to 8000 per cell [223]. The entire mitochondrial genome is dedicated to energy production, and each copy codes for 13 electron transport chain protein subunits necessary for oxidative phosphorylation (the process of converting ADP to ATP). The rest of the mitochondrial genome includes two ribosomal RNA genes and 22 transfer RNAs that provide translational machinery.
Mitochondria maintain an electrical membrane potential (ΔΨm) that is dependent on oxidative phosphorylation. In the coupled state, ΔΨm is maintained at a resting potential between −180 and −200 mV due to the serial reduction of electrons within the inner membrane [224,225]. This electrical potential is required for the import of mitochondrial proteins from the cytosol. It is also necessary for ATP transport out of the mitochondria and is coupled to the activity of the mitochondrial permeability transition pore, which has been shown to regulate the exchange of metabolites and ions such as calcium (involved in apoptosis) [226-228].
During aging and in response to HIV-1 infection and HAART treatment, virtually all aspects of mitochondrial function may be impaired (Table 2) [229,230]. Due to the direct impact of NRTIs, mtDNA deletions and mutations have been examined as a potential cause of HIV-1- and HAART-related pathologies. During aging, mutations in mtDNA, in the form of large deletions, accumulate in a variety of tissues [230]. The most recent examination of this issue, in the aging primate liver, showed increased mtDNA lesions and increased oxidative stress in mononucleocytes with age [231]. In addition, mice bearing a defective allele of the mtDNA polymerase-γ exhibit a premature aging phenotype [232]. In the case of HIV and HAART, the NRTIs directly inhibit DNA polymerase-γ, although the ability of the enzyme to distinguish and remove these compounds from the nascent DNA chain varies [233,234]. Attempts to quantify the impact of NRTIs on the mitochondrial genome in the clinic have taken several forms. In most cases, peripheral blood cells have been examined, in the hope that mitochondrial changes in this cell population would reflect changes associated with pathologies related to mitochondrial dysfunction, such as lipodystrophy and metabolic changes. Results have been conflicting, with some studies reporting a decline in mtDNA content and others failing to find such an association, as previously reviewed by Curran and Ribera [235]. Possible causes for variation between multiple studies include heterogeneous patient genetic backgrounds and the fact that lipodystrophy is almost certainly a multifactorial syndrome. Indeed, a recent study found that patients harboring a polymorphism at E1143 of the polymerase-γ gene have a fourfold higher risk for the development of lipodystrophy while on ART [236]. Haplogroups of mtDNA were associated with differences in metabolic response to ART in an independent study [237], although the development of lipoatrophy was not related to mitochondrial haplogroups in a separate study [238]. Individuals probably vary in susceptibility to the influence of a specific therapy on the mitochondrial genome, and the rate of expansion for mitochondrial mutations also appears to vary. A variation in mitochondrial mutations during aging has long been recognized [239,240]. The variability in the rate of expansion of mtDNA mutations in individuals on HAART was demonstrated in a recent report examining mtDNA deletions and the expression of cytochrome C oxidase subunits in individual muscle fibers [241]. Thus, an individualized approach to the issue of mitochondrial toxicity in ART will be needed to resolve the problems faced in the clinic.
Conclusion
Aging within the context of HIV-1 disease is a complicated and expanding area of research. Age-related diseases such as bone disease metabolic disorders, cardiovascular diseases, and immunologic dysregulation are linked to HIV-1 infection in various ways. HIV-1 infection can lead to a premature aging phenotype, and disease and treatment effects are different in patients who are infected at older ages. The effects of age on HIV-1 infection and markers of HIV-1 disease progression are controversial. The literature varies on the effects age has on VL and on CD4+ T-cell counts in older HAART-treated patients. More research is needed to completely elucidate the effects age has on HIV-1 disease and how best to treat these patients.
Future perspective
Studies on the impact of HIV-1 infection and age on each other have often been discrepant or did not account for possible confounding factors. With regard to the effects of age on CD4+ T-cell count and VL, comparing the overall mortality of older versus younger patients may be most useful. A study examining 788 patients (253 older and 535 younger) showed that in patients not receiving HAART, the hazard rate for death for older patients was twice that observed in younger patients. However, after the initiation of HAART, mortality was reduced by 72% among the older patients, and this reduction increased with HAART usage until there was no statistical difference between the two groups [160]. These results suggest that deferring treatment in older patients, as well as failing to diagnose infection, may adversely impact survival. Failing to diagnose infection in older patients is especially problematic. Older patients are less likely to be tested for HIV-1 infection and are more likely to be misdiagnosed than younger patients [242-246]. There is a misperception that older patients are less at risk for HIV infection, and many of the symptoms, including fatigue, frailty and increased illness, may be misattributed to increased age itself. Thus, older patients are typically diagnosed much later in the course of infection than younger patients.
Regarding neurocognitive impairment in HIV infection, a variety of methodological and scientific issues must be considered. First, for this population, an age cutoff demarcating younger versus older patients may be arbitrary and could be less optimal than treating age as a continuous variable. The patient’s premorbid neurocognitive status should be assessed if possible. Cognitive reserve may have protective effects. Genetic factors such as APOE status have been shown to convey risk for mild cognitive impairment in middle-aged and older patients not infected with HIV-1 [247]. There is no reason why a similar situation would not exist for HIV-1-infected patients. Operationally defining cerebrovascular risk with tools such as the Framingham Stroke Risk Index has been shown to predict both the presence and subtype of mild cognitive impairment in patients without HIV-1 infection [247]. These measures could be similarly useful in older HIV-1-infected patients. Regarding possible links between mitochondrial effects and neurocognitive decline, the challenge is to identify parameters that will reflect differences in mitochondrial function/biogenesis/homeostasis in specific regions of the brain in individuals. This might permit quantifying an individual’s relative susceptibility to specific therapies. This information would allow a relative assessment of the risk of cognitive decline.
In conclusion, HIV-1 infection and aging itself both appear to play important roles in the increased morbidity and mortality of older HIV-1-infected patients. A complex interplay occurs between these factors and HIV-1 infection and subsequent immune dysregulation. HIV-1 infection also appears to cause a state of premature aging, in which infected patients not only develop disorders and diseases that are typically thought of as age-related, but also present with immune dysfunction similar to that observed in elderly uninfected patients. These conditions include neurocognitive impairment, bone disease, diabetes and other metabolic disorders and cardiovascular diseases, among others. In addition to occurring earlier, these conditions also appear to be more aggressive and progress more rapidly in HIV-1-infected patients when compared with the general population [110]. Many HIV-1-infected patients suffer from more than one of these conditions associated with accelerated aging, known as polypathology. Polypathology is also seen in elderly individuals as part of the general population, but it has been shown to occur approximately 15 years earlier in HIV-1-infected patients. These conditions, both singly and combined as polypathologies, appear to be exacerbated not only by HIV-1 infection itself, but also by treatment. To date, the most explored hypotheses for premature aging include the development of mitochondrial toxicity and immunosenescence [68,248]. Although research does suggest a significant role for HAART in premature aging, the benefits of HAART are incontrovertible, and the link between aging-related phenotypes and HIV-1 infection (in the absence or presence of HAART) means that we should focus on the early diagnosis and treatment of infected patients. We hope that early control of VL and CD4+ T-cell counts will help to minimize the premature onset of age-related diseases. Finally, developing next-generation compounds for HIV-1 treatment with decreased toxicity continues to be a high priority to minimize the relationship between HIV-1 infection and aging-related morbidities.
Executive summary.
Aging-related comorbidities within the HIV-1-infected population
-
■
The prevalence of multiple aging-related diseases in HIV-1-infected patients is equivalent to their prevalence in uninfected patients who are 10–15 years older.
-
■
Diabetes incidence is increased in HIV-1-infected patients, with increasing age being associated with increasing risk, and HAART use demonstrating an even greater association with diabetes.
-
■
The link between HAART and diabetes may indirectly involve peripheral fat wasting (lipoatrophy) and abnormal lipid and lipoprotein profiles (dyslipidemia).
-
■
Premature aging occurs not only in the CD8+ T-cell population, but also in specific subsets of naive CD4+ T cells.
-
■
Immunosenescence associated with HIV-1 contributes to long-term immunodeficiency and premature aging diseases.
-
■
HIV-1 proteins, as well as some inflammatory mediators upregulated by HIV-1 infection, have been shown to increase osteoclast activity and promote osteoporosis.
-
■
Appropriate screening times and treatment efficacy for bone loss in HIV-1-infected patients need to be assessed in this growing population.
-
■
A growing body of research demonstrates that neuropathological markers normally associated with Alzheimer’s disease are also present in HIV-1 neurocognitive diseases.
-
■
Traditional risk factors for dementia, including smoking, dyslipidemia, hypertension and diabetes, are increased in HIV-1-infected patients and can be associated with greater cognitive impairment.
Effects of aging on markers of HIV-1 disease progression
-
■
Controversy exists with regard to virological response to HAART, with some studies finding no difference between younger and older HIV-1-infected patients and others finding a better response in older patients.
-
■
Studies have demonstrated an inverse relationship between CD4+ T-cell count and patient age; however, this finding is controversial, with other studies demonstrating no connection between age and response to HAART with regard to CD4+ T-cell count.
-
■
The increased virologic response observed in some older HIV-1-infected patients, as well as the result of no change between younger and older HIV-1-infected patients with regard to CD4+ T-cell response, may be the result of increased adherence to HAART.
-
■
HIV-1 infection results in decreased function of macrophages, as is seen with aging.
-
■
HIV-1 infection, as well as increasing age, result in an increase in basal plasma levels of IL-6, as well as increases in p55- and p75-soluble TNF-α receptor.
-
■
Virtually all aspects of mitochondrial function may be impaired during aging and in response to HIV-1 infection and HAART treatment.
-
■
Mice bearing a defective allele of the mitochondrial DNA polymerase-γ exhibit a premature aging phenotype, and nucleoside reverse transcriptase inhibitors have also been shown to directly inhibit DNA polymerase-γ.
Future perspective
-
■
The impact of mortality of older HIV-1-infected patients in comparison with younger HIV-1-infected patients may be more beneficial with regard to determining the effect of age on both CD4+ T-cell count and viral load.
-
■
Age cutoff, premorbid neurocognitive status, genetic factors, cardiovascular risk and mitochondrial risks must be considered in the assessment of age-related neurocognitive impairment in HIV-1.
-
■
Continued production of next-generation compounds for HIV-1 treatment with decreased toxicity should be a high priority.
Acknowledgments
V Pirrone, MR Nonnemacher and B Wigdahl are supported in part by funds from the Public Health Service, NIH, through grants from the National Institute of Neurological Disorders and Stroke, NS32092 and NS46263 (B Wigdahl, Principal Investigator) and the National Institute of Drug Abuse, DA19807 (B Wigdahl, Principal Investigator). MR Nonnemacher is also supported by research developmental funding provided by the Department of Microbiology and Immunology and the Institute for Molecular Medicine and Infectious Disease, Drexel University College of Medicine. C Sell and CA Lerner are supported in part by funds from the NIH through a grant from the National Institute of Aging, AG39799 (C Sell, Principal Investigator). DJ Libon is supported in part by funds from the NIH, National Institute on Aging/National Institute for Neurological Disease NS053488 and National Institute on Aging AG032953, AG017586 and NS044266.
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
■ of interest
■■ of considerable interest
- 1.Hogg RS, Heath KV, Yip B, et al. Improved survival among HIV-infected individuals following initiation of antiretroviral therapy. JAMA. 1998;279(6):450–454. doi: 10.1001/jama.279.6.450. [DOI] [PubMed] [Google Scholar]
- 2.Hogg RS, O’Shaughnessy MV, Gataric N, et al. Decline in deaths from AIDS due to new antiretrovirals. Lancet. 1997;349(9061):1294. doi: 10.1016/S0140-6736(05)62505-6. [DOI] [PubMed] [Google Scholar]
- 3.Sterne JA, Hernan MA, Ledergerber B, et al. Long-term effectiveness of potent antiretroviral therapy in preventing AIDS and death: a prospective cohort study. Lancet. 2005;366(9483):378–384. doi: 10.1016/S0140-6736(05)67022-5. [DOI] [PubMed] [Google Scholar]
- 4.Wong KH, Chan KC, Lee SS. Delayed progression to death and to AIDS in a Hong Kong cohort of patients with advanced HIV type 1 disease during the era of highly active antiretroviral therapy. Clin. Infect. Dis. 2004;39(6):853–860. doi: 10.1086/423183. [DOI] [PubMed] [Google Scholar]
- 5.Smith G. Aging Hearing: HIV Over Fifty, Exploring the New Threat. US Government Printing Office; DC, USA: 2006. [Google Scholar]
- 6.Lohse N, Hansen AB, Pedersen G, et al. Survival of persons with and without HIV infection in Denmark, 1995–2005. Ann. Intern. Med. 2007;146(2):87–95. doi: 10.7326/0003-4819-146-2-200701160-00003. [DOI] [PubMed] [Google Scholar]
- 7.Lewden C, Chene G, Morlat P, et al. HIV-infected adults with a CD4 cell count greater than 500 cells/mm3 on long-term combination antiretroviral therapy reach same mortality rates as the general population. J. Acquir. Immune Defic. Syndr. 2007;46(1):72–77. doi: 10.1097/QAI.0b013e318134257a. [DOI] [PubMed] [Google Scholar]
- 8.Hogg R, Lima V, Sterne JA, et al. Life expectancy of individuals on combination antiretroviral therapy in high-income countries: a collaborative analysis of 14 cohort studies. Lancet. 2008;372(9635):293–299. doi: 10.1016/S0140-6736(08)61113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goulet JL, Fultz SL, Rimland D, et al. Aging and infectious diseases: do patterns of comorbidity vary by HIV status, age, and HIV severity? Clin. Infect. Dis. 2008;45(12):1593–1601. doi: 10.1086/523577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bisson G, Gross R, Miller V, et al. Monitoring of long-term toxicities of HIV treatments: an international perspective. AIDS. 2003;17(17):2407–2417. doi: 10.1097/00002030-200311210-00002. [DOI] [PubMed] [Google Scholar]
- 11.Gress RE, Deeks SG. Reduced thymus activity and infection prematurely age the immune system. J. Clin. Invest. 2009;119(10):2884–2887. doi: 10.1172/JCI40855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deeks SG. Immune dysfunction, inflammation, and accelerated aging in patients on antiretroviral therapy. Top. HIV Med. 2009;17(4):118–123. [PubMed] [Google Scholar]
- 13.Kirk JB, Goetz MB. Human immunodeficiency virus in an aging population, a complication of success. J. Am. Geriatr. Soc. 2009;57(11):2129–2138. doi: 10.1111/j.1532-5415.2009.02494.x. [DOI] [PubMed] [Google Scholar]
- 14■.Deeks SG, Phillips AN. HIV infection, antiretroviral treatment, ageing, and non-AIDS related morbidity. BMJ. 2009;338:a3172. doi: 10.1136/bmj.a3172. Discusses the clinical implications of successful HAART and the development of age-related diseases.
- 15.Effros RB, Fletcher CV, Gebo K, et al. Aging and infectious diseases: workshop on HIV infection and aging: what is known and future research directions. Clin. Infect. Dis. 2008;47(4):542–553. doi: 10.1086/590150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16■■.Guaraldi G, Orlando G, Zona S, et al. Premature age-related comorbidities among HIV-infected persons compared with the general population. Clin. Infect. Dis. 2011;53(11):1120–1126. doi: 10.1093/cid/cir627. Noninfectious comorbidities and polypathologies typically related to advanced age are more commonly encountered in HIV-1-infected patients. The prevalence of these conditions in HIV-1-infected patients is equivalent to their prevalence in uninfected patients 10 years older.
- 17.Guaraldi G, Zona S, Alexopoulos N, et al. Coronary aging in HIV-infected patients. Clin. Infect. Dis. 2009;49(11):1756–1762. doi: 10.1086/648080. [DOI] [PubMed] [Google Scholar]
- 18.Caron-Debarle M, Lagathu C, Boccara F, Vigouroux C, Capeau J. HIV-associated lipodystrophy: from fat injury to premature aging. Trends Mol. Med. 2010;16(5):218–229. doi: 10.1016/j.molmed.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 19.Deeks SG. HIV infection, inflammation, immunosenescence, and aging. Annu. Rev. Med. 2011;62:141–155. doi: 10.1146/annurev-med-042909-093756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.King H, Aubert RE, Herman WH. Global burden of diabetes, 1995–2025: prevalence, numerical estimates, and projections. Diabetes Care. 1998;21(9):1414–1431. doi: 10.2337/diacare.21.9.1414. [DOI] [PubMed] [Google Scholar]
- 21.Morley JE. The elderly Type 2 diabetic patient: special considerations. Diabetic Med. 1998;15(Suppl. 4):S41–S46. doi: 10.1002/(sici)1096-9136(1998120)15:4+<s41::aid-dia747>3.3.co;2-5. [DOI] [PubMed] [Google Scholar]
- 22.HIV and the pancreas. Lancet. 1987;2(8569):1212–1213. [PubMed] [Google Scholar]
- 23■.Butt AA, McGinnis K, Rodriguez-Barradas MC, et al. HIV infection and the risk of diabetes mellitus. AIDS. 2009;23(10):1227–1234. doi: 10.1097/QAD.0b013e32832bd7af. Increasing age, HCV coinfection and BMI were all found to affect the risk of diabetes in HIV-1-infected patients. Long-term HAART was also found to increase the risk.
- 24.Capeau J. From lipodystrophy and insulin resistance to metabolic syndrome: HIV infection, treatment and aging. Curr. Opin. HIV AIDS. 2007;2(4):247–252. doi: 10.1097/COH.0b013e3281e66919. [DOI] [PubMed] [Google Scholar]
- 25.Grunfeld C, Pang M, Doerrler W, Shigenaga JK, Jensen P, Feingold KR. Lipids, lipoproteins, triglyceride clearance, and cytokines in human immunodeficiency virus infection and the acquired immunodeficiency syndrome. J. Clin. Endocrinol. Metab. 1992;74(5):1045–1052. doi: 10.1210/jcem.74.5.1373735. [DOI] [PubMed] [Google Scholar]
- 26.Brown TT, Cole SR, Li X, et al. Antiretroviral therapy and the prevalence and incidence of diabetes mellitus in the multicenter AIDS cohort study. Arch. Intern. Med. 2005;165(10):1179–1184. doi: 10.1001/archinte.165.10.1179. [DOI] [PubMed] [Google Scholar]
- 27.De Wit S, Sabin CA, Weber R, et al. Incidence and risk factors for new-onset diabetes in HIV-infected patients: the Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D) study. Diabetes Care. 2008;31(6):1224–1229. doi: 10.2337/dc07-2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tien PC, Schneider MF, Cole SR, et al. Antiretroviral therapy exposure and incidence of diabetes mellitus in the Women’s Interagency HIV Study. AIDS. 2007;21(13):1739–1745. doi: 10.1097/QAD.0b013e32827038d0. [DOI] [PubMed] [Google Scholar]
- 29.Cossarizza A, Moyle G. Antiretroviral nucleoside and nucleotide analogues and mitochondria. AIDS. 2004;18(2):137–151. doi: 10.1097/00002030-200401230-00002. [DOI] [PubMed] [Google Scholar]
- 30.Lowell BB, Shulman GI. Mitochondrial dysfunction and Type 2 diabetes. Science. 2005;307(5708):384–387. doi: 10.1126/science.1104343. [DOI] [PubMed] [Google Scholar]
- 31■■.Samaras K. Prevalence and pathogenesis of diabetes mellitus in HIV-1 infection treated with combined antiretroviral therapy. J. Acquir. Immune Defic. Syndr. 2009;50(5):499–505. doi: 10.1097/QAI.0b013e31819c291b. Reviews the prevalence of diabetes in HAART-treated HIV-1-infected patients, as well as potential mechanisms, including insulin resistance.
- 32.Carr A, Samaras K, Burton S, et al. A syndrome of peripheral lipodystrophy, hyperlipidaemia and insulin resistance in patients receiving HIV protease inhibitors. AIDS. 1998;12(7):F51–F58. doi: 10.1097/00002030-199807000-00003. [DOI] [PubMed] [Google Scholar]
- 33.Behrens G, Dejam A, Schmidt H, et al. Impaired glucose tolerance, beta cell function and lipid metabolism in HIV patients under treatment with protease inhibitors. AIDS. 1999;13(10):F63–F70. doi: 10.1097/00002030-199907090-00001. [DOI] [PubMed] [Google Scholar]
- 34.Walli R, Herfort O, Michl GM, et al. Treatment with protease inhibitors associated with peripheral insulin resistance and impaired oral glucose tolerance in HIV-1-infected patients. AIDS. 1998;12(15):F167–F173. doi: 10.1097/00002030-199815000-00001. [DOI] [PubMed] [Google Scholar]
- 35.van der Valk M, Kastelein JJ, Murphy RL, et al. Nevirapine-containing antiretroviral therapy in HIV-1 infected patients results in an anti-atherogenic lipid profile. AIDS. 2001;15(18):2407–2414. doi: 10.1097/00002030-200112070-00008. [DOI] [PubMed] [Google Scholar]
- 36■.van der Valk M, Bisschop PH, Romijn JA, et al. Lipodystrophy in HIV-1-positive patients is associated with insulin resistance in multiple metabolic pathways. AIDS. 2001;15(16):2093–2100. doi: 10.1097/00002030-200111090-00004. Demonstrated that postabsorptive glucose production is increased in HIV-1-infected patients who demonstrate fat redistribution (lipodystrophy).
- 37.van der Valk M, Gisolf EH, Reiss P, et al. Increased risk of lipodystrophy when nucleoside analogue reverse transcriptase inhibitors are included with protease inhibitors in the treatment of HIV-1 infection. AIDS. 2001;15(7):847–855. doi: 10.1097/00002030-200105040-00005. [DOI] [PubMed] [Google Scholar]
- 38.Albu JB, Kovera AJ, Allen L, et al. Independent association of insulin resistance with larger amounts of intermuscular adipose tissue and a greater acute insulin response to glucose in African American than in white nondiabetic women. Am. J. Clin. Nutr. 2005;82(6):1210–1217. doi: 10.1093/ajcn/82.6.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grunfeld C, Rimland D, Gibert CL, et al. Association of upper trunk and visceral adipose tissue volume with insulin resistance in control and HIV-infected subjects in the FRAM study. J. Acquir. Immune Defic. Syndr. 2008;46(3):283–290. doi: 10.1097/qai.0b013e31814b94e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stanley TL, Grinspoon SK. Body composition and metabolic changes in HIV-infected patients. J. Infect. Dis. 2012;205(Suppl. 3):S383–S390. doi: 10.1093/infdis/jis205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wohl D, Scherzer R, Heymsfield S, et al. The associations of regional adipose tissue with lipid and lipoprotein levels in HIV-infected men. J. Acquir. Immune Defic. Syndr. 2008;48(1):44–52. doi: 10.1097/QAI.0b013e31816d9ba1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wohl DA, Brown TT. Management of morphologic changes associated with antiretroviral use in HIV-infected patients. J. Acquir. Immune Defic. Syndr. 2008;49(Suppl. 2):S93–S100. doi: 10.1097/QAI.0b013e318186521a. [DOI] [PubMed] [Google Scholar]
- 43.McLaughlin T, Lamendola C, Liu A, Abbasi F. Preferential fat deposition in subcutaneous versus visceral depots is associated with insulin sensitivity. J. Clin. Endocrinol. Metab. 2011;96(11):E1756–E1760. doi: 10.1210/jc.2011-0615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hadigan C, Borgonha S, Rabe J, Young V, Grinspoon S. Increased rates of lipolysis among human immunodeficiency virus-infected men receiving highly active antiretroviral therapy. Metabolism. 2002;51(9):1143–1147. doi: 10.1053/meta.2002.34704. [DOI] [PubMed] [Google Scholar]
- 45■.Shlay JC, Visnegarwala F, Bartsch G, et al. Body composition and metabolic changes in antiretroviral-naive patients randomized to didanosine and stavudine vs. abacavir and lamivudine. J. Acquir. Immune Defic. Syndr. 2005;38(2):147–155. doi: 10.1097/01.qai.0000143599.64234.15. Compared body composition and metabolic changes among HAART-naive patients randomly assigned to either a didanosine plus stavudine regimen or an abacavir plus lamivudine regimen. Early and sustained increases in both insulin and insulin resistance were seen only in the didanosine plus stavudine regimen.
- 46.Lowe SH, Hassink EA, van Eck-Smit BL, Borleffs JC, Lange JM, Reiss P. Stavudine but not didanosine as part of HAART contributes to peripheral lipoatrophy: a substudy from the Antiretroviral Regimen Evaluation Study (ARES) HIV Clin. Trials. 2007;8(5):337–344. doi: 10.1310/hct0805-337. [DOI] [PubMed] [Google Scholar]
- 47.Feeney ER, Mallon PW. HIV and HAART-associated dyslipidemia. Open Cardiovasc. Med. J. 2011;5:49–63. doi: 10.2174/1874192401105010049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grunfeld C, Kotler DP, Hamadeh R, Tierney A, Wang J, Pierson RN. Hypertriglyceridemia in the acquired immunodeficiency syndrome. Am. J. Med. 1989;86(1):27–31. doi: 10.1016/0002-9343(89)90225-8. [DOI] [PubMed] [Google Scholar]
- 49.Riddler SA, Smit E, Cole SR, et al. Impact of HIV infection and HAART on serum lipids in men. JAMA. 2003;289(22):2978–2982. doi: 10.1001/jama.289.22.2978. [DOI] [PubMed] [Google Scholar]
- 50.Shor-Posner G, Basit A, Lu Y, et al. Hypocholesterolemia is associated with immune dysfunction in early human immunodeficiency virus-1 infection. Am. J. Med. 1993;94(5):515–519. doi: 10.1016/0002-9343(93)90087-6. [DOI] [PubMed] [Google Scholar]
- 51.Anastos K, Lu D, Shi Q, et al. Association of serum lipid levels with HIV serostatus, specific antiretroviral agents, and treatment regimens. J. Acquir. Immune Defic. Syndr. 2007;45(1):34–42. doi: 10.1097/QAI.0b013e318042d5fe. [DOI] [PubMed] [Google Scholar]
- 52.El-Sadr WM, Mullin CM, Carr A, et al. Effects of HIV disease on lipid, glucose and insulin levels: results from a large antiretroviral-naive cohort. HIV Med. 2005;6(2):114–121. doi: 10.1111/j.1468-1293.2005.00273.x. [DOI] [PubMed] [Google Scholar]
- 53.Rose H, Woolley I, Hoy J, et al. HIV infection and high-density lipoprotein: the effect of the disease vs the effect of treatment. Metabolism. 2005;55(1):90–95. doi: 10.1016/j.metabol.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 54.Barbaro G. Metabolic and cardiovascular complications of highly active antiretroviral therapy for HIV infection. Curr. HIV Res. 2006;4(1):79–85. doi: 10.2174/157016206775197664. [DOI] [PubMed] [Google Scholar]
- 55.Carr A, Samaras K, Chisholm DJ, Cooper DA. Abnormal fat distribution and use of protease inhibitors. Lancet. 1998;351(9117):1736. doi: 10.1016/S0140-6736(05)77775-8. [DOI] [PubMed] [Google Scholar]
- 56.Carr A, Samaras K, Chisholm DJ, Cooper DA. Pathogenesis of HIV-1-protease inhibitor-associated peripheral lipodystrophy, hyperlipidaemia, and insulin resistance. Lancet. 1998;351(9119):1881–1883. doi: 10.1016/S0140-6736(98)03391-1. [DOI] [PubMed] [Google Scholar]
- 57.Walford RL. The immunologic theory of aging. Gerontologist. 1964;57:195–197. doi: 10.1093/geront/4.4.195. [DOI] [PubMed] [Google Scholar]
- 58■■.Dock JN, Effros RB. Role of CD8 T cell replicative senescence in human aging and in HIV-mediated immunosenescence. Aging Dis. 2012;2(5):382–397. Reviews the role of CD8+ T-cell replicative senescence both in vitro and in vivo with respect to human aging and HIV-1 infection.
- 59.Goodwin K, Viboud C, Simonsen L. Antibody response to influenza vaccination in the elderly: a quantitative review. Vaccine. 2005;24(8):1159–1169. doi: 10.1016/j.vaccine.2005.08.105. [DOI] [PubMed] [Google Scholar]
- 60.Hakim FT, Gress RE. Thymic involution: implications for self-tolerance. Methods Mol. Biol. 2007;380:377–390. doi: 10.1007/978-1-59745-395-0_24. [DOI] [PubMed] [Google Scholar]
- 61.Ostan R, Bucci L, Capri M, et al. Immunosenescence and immunogenetics of human longevity. Neuroimmunomodulation. 2008;15(4-6):224–240. doi: 10.1159/000156466. [DOI] [PubMed] [Google Scholar]
- 62.Aspinall R, Del Giudice G, Effros RB, Grubeck-Loebenstein B, Sambhara S. Challenges for vaccination in the elderly. Immun. Ageing. 2007;4:9. doi: 10.1186/1742-4933-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lindstrom TM, Robinson WH. Rheumatoid arthritis: a role for immunosenescence? J. Am. Geriatr. Soc. 2010;58(8):1565–1575. doi: 10.1111/j.1532-5415.2010.02965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pawelec G, Hirokawa K, Fulop T. Altered T cell signalling in ageing. Mech. Ageing Dev. 2001;122(14):1613–1637. doi: 10.1016/s0047-6374(01)00290-1. [DOI] [PubMed] [Google Scholar]
- 65.Plonquet A, Bastuji-Garin S, Tahmasebi F, et al. Immune risk phenotype is associated with nosocomial lung infections in elderly in-patients. Immun. Ageing. 2011;8:8. doi: 10.1186/1742-4933-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ferguson FG, Wikby A, Maxson P, Olsson J, Johansson B. Immune parameters in a longitudinal study of a very old population of Swedish people: a comparison between survivors and nonsurvivors. J. Gerontol. A Biol. Sci. Med. Sci. 1995;50(6):B378–B382. doi: 10.1093/gerona/50a.6.b378. [DOI] [PubMed] [Google Scholar]
- 67.Strindhall J, Nilsson BO, Lofgren S, et al. No Immune Risk Profile among individuals who reach 100 years of age: findings from the Swedish NONA immune longitudinal study. Exp. Gerontol. 2007;42(8):753–761. doi: 10.1016/j.exger.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 68■■.Rickabaugh TM, Kilpatrick RD, Hultin LE, et al. The dual impact of HIV-1 infection and aging on naive CD4 T-cells: additive and distinct patterns of impairment. PLoS One. 2011;6(1):e16459. doi: 10.1371/journal.pone.0016459. Examined subsets of naive CD4+ T cells in HAART-naive, HIV-1-infected patients, comparing younger and older patients. Longitudinal analysis demonstrated differences in thymic emigration and reconstitution of differential subsets 2 years after the initiation of HAART.
- 69■.Molina-Pinelo S, Vallejo A, Diaz L, et al. Premature immunosenescence in HIV-infected patients on highly active antiretroviral therapy with low-level CD4 T cell repopulation. J. Antimicrob. Chemother. 2009;64(3):579–588. doi: 10.1093/jac/dkp248. Demonstrated lower thymic activity in patients with lower levels of T-cell reconstitution. A higher rate of activation and replicative senescence of CD4+ T cells led to a higher rate of apoptotic CD4+ T cells in this population.
- 70.Teixeira L, Valdez H, McCune JM, et al. Poor CD4 T cell restoration after suppression of HIV-1 replication may reflect lower thymic function. AIDS. 2001;15(14):1749–1756. doi: 10.1097/00002030-200109280-00002. [DOI] [PubMed] [Google Scholar]
- 71■.Robbins GK, Spritzler JG, Chan ES, et al. Incomplete reconstitution of T cell subsets on combination antiretroviral therapy in the AIDS Clinical Trials Group protocol 384. Clin. Infect. Dis. 2009;48(3):350–361. doi: 10.1086/595888. Determined a correlation between baseline CD4+ T-cell count at initiation of HAART and the ability to approach levels seen in uninfected patients. Suggests a benefit of starting HAART at levels of 350 cells/mm3 or higher.
- 72.Cao W, Jamieson BD, Hultin LE, Hultin PM, Effros RB, Detels R. Premature aging of T cells is associated with faster HIV-1 disease progression. J. Acquir. Immune Defic. Syndr. 2009;50(2):137–147. doi: 10.1097/QAI.0b013e3181926c28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Phillips AN, Neaton J, Lundgren JD. The role of HIV in serious diseases other than AIDS. AIDS. 2008;22(18):2409–2418. doi: 10.1097/QAD.0b013e3283174636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Consensus development conference: diagnosis, prophylaxis, and treatment of osteoporosis. Am. J. Med. 1993;94(6):646–650. doi: 10.1016/0002-9343(93)90218-e. [DOI] [PubMed] [Google Scholar]
- 75.WHO Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO Study Group. World Health Organ. Tech. Rep. Ser. 1994;843:1–129. [PubMed] [Google Scholar]
- 76.Marshall D, Johnell O, Wedel H. Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ. 1996;312(7041):1254–1259. doi: 10.1136/bmj.312.7041.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bruera D, Luna N, David DO, Bergoglio LM, Zamudio J. Decreased bone mineral density in HIV-infected patients is independent of antiretroviral therapy. AIDS. 2003;17(13):1917–1923. doi: 10.1097/00002030-200309050-00010. [DOI] [PubMed] [Google Scholar]
- 78.Yin M, Dobkin J, Brudney K, et al. Bone mass and mineral metabolism in HIV+ postmenopausal women. Osteoporos. Int. 2005;16(11):1345–1352. doi: 10.1007/s00198-005-1845-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jones S, Restrepo D, Kasowitz A, et al. Risk factors for decreased bone density and effects of HIV on bone in the elderly. Osteoporos. Int. 2007;19(7):913–918. doi: 10.1007/s00198-007-0524-8. [DOI] [PubMed] [Google Scholar]
- 80.Berg KM, Klein RS, Schoenbaum EE, Arnsten JH. Interpreting the association between HIV and bone mineral density. AIDS. 2007;21(6):785–786. doi: 10.1097/QAD.0b013e3280d6ee06. [DOI] [PubMed] [Google Scholar]
- 81.Arnsten JH, Freeman R, Howard AA, Floris-Moore M, Lo Y, Klein RS. Decreased bone mineral density and increased fracture risk in aging men with or at risk for HIV infection. AIDS. 2007;21(5):617–623. doi: 10.1097/QAD.0b013e3280148c05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Carr A, Miller J, Eisman JA, Cooper DA. Osteopenia in HIV-infected men: association with asymptomatic lactic acidemia and lower weight pre-antiretroviral therapy. AIDS. 2001;15(6):703–709. doi: 10.1097/00002030-200104130-00005. [DOI] [PubMed] [Google Scholar]
- 83.Dolan SE, Huang JS, Killilea KM, Sullivan MP, Aliabadi N, Grinspoon S. Reduced bone density in HIV-infected women. AIDS. 2004;18(3):475–483. doi: 10.1097/00002030-200402200-00014. [DOI] [PubMed] [Google Scholar]
- 84.Knobel H, Guelar A, Vallecillo G, Nogues X, Diez A. Osteopenia in HIV-infected patients: is it the disease or is it the treatment? AIDS. 2001;15(6):807–808. doi: 10.1097/00002030-200104130-00022. [DOI] [PubMed] [Google Scholar]
- 85.Tebas P, Powderly WG, Claxton S, et al. Accelerated bone mineral loss in HIV-infected patients receiving potent antiretroviral therapy. AIDS. 2000;14(4):F63–F67. doi: 10.1097/00002030-200003100-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Teichmann J, Stephan E, Lange U, et al. Osteopenia in HIV-infected women prior to highly active antiretroviral therapy. J. Infect. 2003;46(4):221–227. doi: 10.1053/jinf.2002.1109. [DOI] [PubMed] [Google Scholar]
- 87.Triant VA, Brown TT, Lee H, Grinspoon SK. Fracture prevalence among human immunodeficiency virus (HIV)-infected versus non-HIV-infected patients in a large U.S. healthcare system. J. Clin. Endocrinol. Metab. 2008;93(9):3499–3504. doi: 10.1210/jc.2008-0828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dao C, Young B, Buchacz K, et al. Higher and increasing rates of fracture among HIV-infected persons in the HIV Outpatient Study (HOPS) compared to the general US population, 1994 to 2008; Presented at: 17th Conference on Retroviruses and Opportunistic Infections; San Francisco, CA, USA. 2010.Feb 16-19, [Google Scholar]
- 89.Womack J, Goulet JL, Gibert C, et al. HIV-infection and fragility fracture risk among male veterans; Presented at: 17th Conference on Retroviruses and Opportunistic Infections; San Francisco, CA, USA. 2010.Feb 16-19, [Google Scholar]
- 90.Mondy K, Tebas P. Emerging bone problems in patients infected with human immunodeficiency virus. Clin. Infect. Dis. 2003;36(Suppl. 2):S101–S105. doi: 10.1086/367566. [DOI] [PubMed] [Google Scholar]
- 91.Mondy K, Yarasheski K, Powderly WG, et al. Longitudinal evolution of bone mineral density and bone markers in human immunodeficiency virus-infected individuals. Clin. Infect. Dis. 2003;36(4):482–490. doi: 10.1086/367569. [DOI] [PubMed] [Google Scholar]
- 92.Gallant JE, Staszewski S, Pozniak AL, et al. Efficacy and safety of tenofovir DF vs stavudine in combination therapy in antiretroviral-naive patients: a 3-year randomized trial. JAMA. 2004;292(2):191–201. doi: 10.1001/jama.292.2.191. [DOI] [PubMed] [Google Scholar]
- 93■.Gibellini D, De Crignis E, Ponti C, et al. HIV-1 triggers apoptosis in primary osteoblasts and HOBIT cells through TNFalpha activation. J. Med. Virol. 2008;80(9):1507–1514. doi: 10.1002/jmv.21266. Demonstrated a link between TNF-α upregulation by HIV-1 infection and the initiation of apoptosis in primary osteoblasts and HOBIT cells, suggesting a link between HIV-1 infection and bone disease.
- 94.Bismar H, Diel I, Ziegler R, Pfeilschifter J. Increased cytokine secretion by human bone marrow cells after menopause or discontinuation of estrogen replacement. J. Clin. Endocrinol. Metab. 1995;80(11):3351–3355. doi: 10.1210/jcem.80.11.7593450. [DOI] [PubMed] [Google Scholar]
- 95■.Brown TT, Qaqish RB. Antiretroviral therapy and the prevalence of osteopenia and osteoporosis: a meta-analytic review. AIDS. 2006;20(17):2165–2174. doi: 10.1097/QAD.0b013e32801022eb. Showed that the prevalence of osteoporosis in HIV-1-infected patients was three-times greater than in uninfected controls. In addition, those treated with protease inhibitors demonstrated decreased bone mass density (BMD) and increased odds of osteoporosis compared with those not treated with protease inhibitors.
- 96■■.Brown TT, McComsey GA, King MS, Qaqish RB, Bernstein BM, da Silva BA. Loss of bone mineral density after antiretroviral therapy initiation, independent of antiretroviral regimen. J. Acquir. Immune Defic. Syndr. 2009;51(5):554–561. doi: 10.1097/QAI.0b013e3181adce44. Found a similar decrease in BMD in HAART-naive patients receiving either efavirenz or lopinavir/ritonavir-based regimens. This was not altered by lopinavir/ritonavir monotherapy and was found to be unrelated to TNF-α activity.
- 97.Duvivier C, Kolta S, Assoumou L, et al. Greater decrease in bone mineral density with protease inhibitor regimens compared with nonnucleoside reverse transcriptase inhibitor regimens in HIV-1 infected naive patients. AIDS. 2009;23(7):817–824. doi: 10.1097/QAD.0b013e328328f789. [DOI] [PubMed] [Google Scholar]
- 98.Ellfolk M, Norlin M, Gyllensten K, Wikvall K. Regulation of human vitamin D3 25-hydroxylases in dermal fibroblasts and prostate cancer LNCaP cells. Mol. Pharmacol. 2009;75(6):1392–1399. doi: 10.1124/mol.108.053660. [DOI] [PubMed] [Google Scholar]
- 99.Fabbriciani G, De Socio GV. Efavirenz and bone health. AIDS. 2009;23(9):1181. doi: 10.1097/QAD.0b013e32832bab0f. [DOI] [PubMed] [Google Scholar]
- 100.Herzmann C, Arasteh K. Efavirenz-induced osteomalacia. AIDS. 2008;23(2):274–275. doi: 10.1097/QAD.0b013e32831f4685. [DOI] [PubMed] [Google Scholar]
- 101.Landriscina M, Altamura SA, Roca L, et al. Reverse transcriptase inhibitors induce cell differentiation and enhance the immunogenic phenotype in human renal clear-cell carcinoma. Int. J. Cancer. 2008;122(12):2842–2850. doi: 10.1002/ijc.23197. [DOI] [PubMed] [Google Scholar]
- 102.Mouly S, Lown KS, Kornhauser D, et al. Hepatic but not intestinal CYP3A4 displays dose-dependent induction by efavirenz in humans. Clin. Pharmacol. Ther. 2002;72(1):1–9. doi: 10.1067/mcp.2002.124519. [DOI] [PubMed] [Google Scholar]
- 103.Bolland MJ, Grey AB, Horne AM, et al. Bone mineral density is not reduced in HIV-infected Caucasian men treated with highly active antiretroviral therapy. Clin. Endocrinol. 2006;65(2):191–197. doi: 10.1111/j.1365-2265.2006.02572.x. [DOI] [PubMed] [Google Scholar]
- 104.Dolan SE, Kanter JR, Grinspoon S. Longitudinal analysis of bone density in human immunodeficiency virus-infected women. J. Clin. Endocrinol. Metab. 2006;91(8):2938–2945. doi: 10.1210/jc.2006-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Spector SA. Vitamin D and HIV: letting the sun shine in. Top. Antivir. Med. 2011;19(1):6–10. [PMC free article] [PubMed] [Google Scholar]
- 106.McComsey GA, Kendall MA, Tebas P, et al. Alendronate with calcium and vitamin D supplementation is safe and effective for the treatment of decreased bone mineral density in HIV. AIDS. 2007;21(18):2473–2482. doi: 10.1097/QAD.0b013e3282ef961d. [DOI] [PubMed] [Google Scholar]
- 107■.Yin MT, McMahon DJ, Ferris DC, et al. Low bone mass and high bone turnover in postmenopausal human immunodeficiency virus-infected women. J. Clin. Endocrinol. Metab. 2009;95(2):620–629. doi: 10.1210/jc.2009-0708. Determined that HIV-1-infected postmenopausal minority women had lower BMD, higher prevalence of low BMD and higher levels of bone turnover markers, which can put them at risk for increased fractures.
- 108.Ritchie K, Touchon J. Mild cognitive impairment: conceptual basis and current nosological status. Lancet. 2000;355(9199):225–228. doi: 10.1016/S0140-6736(99)06155-3. [DOI] [PubMed] [Google Scholar]
- 109.Levy R. Aging-associated cognitive decline. Working Party of the International Psychogeriatric Association in collaboration with the World Health Organization. Int. Psychogeriatr. 1994;6(1):63–68. [PubMed] [Google Scholar]
- 110.Bhatia R, Ryscavage P, Taiwo B. Accelerated aging and human immunodeficiency virus infection: emerging challenges of growing older in the era of successful antiretroviral therapy. J. Neurovirol. 2011;18(4):247–255. doi: 10.1007/s13365-011-0073-y. [DOI] [PubMed] [Google Scholar]
- 111.Antinori A, Arendt G, Becker JT, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69(18):1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112■■.Valcour V, Shikuma C, Shiramizu B, et al. Higher frequency of dementia in older HIV-1 individuals: the Hawaii Aging with HIV-1 Cohort. Neurology. 2004;63(5):822–827. doi: 10.1212/01.wnl.0000134665.58343.8d. Within the Hawaii Aging with HIV-1 cohort, older age was associated with increased HIV-associated dementia after adjusting for a number of important factors.
- 113.Valcour V, Shikuma C, Shiramizu B, et al. Age, apolipoprotein E4, and the risk of HIV dementia: the Hawaii Aging with HIV Cohort. J. Neuroimmunol. 2004;157(1-2):197–202. doi: 10.1016/j.jneuroim.2004.08.029. [DOI] [PubMed] [Google Scholar]
- 114.Becker JT, Kingsley L, Mullen J, et al. Vascular risk factors, HIV serostatus, and cognitive dysfunction in gay and bisexual men. Neurology. 2009;73(16):1292–1299. doi: 10.1212/WNL.0b013e3181bd10e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wright EJ, Grund B, Robertson K, et al. Cardiovascular risk factors associated with lower baseline cognitive performance in HIV-positive persons. Neurology. 2010;75(10):864–873. doi: 10.1212/WNL.0b013e3181f11bd8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116■■.Becker JT, Lopez OL, Dew MA, Aizenstein HJ. Prevalence of cognitive disorders differs as a function of age in HIV virus infection. AIDS. 2004;18(Suppl. 1):S11–S18. Demonstrated that the prevalence of cognitive disorders was higher among older HIV-1-infected patients compared with younger patients. Dementia was the most common classification in the older group, with milder forms of neurocognitive impairment more common among the younger group.
- 117.Anthony IC, Ramage SN, Carnie FW, Simmonds P, Bell JE. Accelerated tau deposition in the brains of individuals infected with human immunodeficiency virus-1 before and after the advent of highly active antiretroviral therapy. Acta Neuropathol. 2006;111(6):529–538. doi: 10.1007/s00401-006-0037-0. [DOI] [PubMed] [Google Scholar]
- 118.Khanlou N, Moore DJ, Chana G, et al. Increased frequency of alpha-synuclein in the substantia nigra in human immunodeficiency virus infection. J. Neurovirol. 2008;15(2):131–138. doi: 10.1080/13550280802578075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Brew BJ, Pemberton L, Blennow K, Wallin A, Hagberg L. CSF amyloid beta42 and tau levels correlate with AIDS dementia complex. Neurology. 2005;65(9):1490–1492. doi: 10.1212/01.wnl.0000183293.95787.b7. [DOI] [PubMed] [Google Scholar]
- 120.Clifford DB, Fagan AM, Holtzman DM, et al. CSF biomarkers of Alzheimer disease in HIV-associated neurologic disease. Neurology. 2009;73(23):1982–1987. doi: 10.1212/WNL.0b013e3181c5b445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121■.Valcour V, Watters MR, Williams AE, Sacktor N, McMurtray A, Shikuma C. Aging exacerbates extrapyramidal motor signs in the era of highly active antiretroviral therapy. J. Neurovirol. 2008;14(5):362–367. doi: 10.1080/13550280802216494. Concluded that extrapyramidal motor signs were increased in HAART-treated HIV-1-infected patients, and this effect was exacerbated by aging.
- 122.Sacktor N, Lyles RH, Skolasky R, et al. HIV-associated neurologic disease incidence changes: Multicenter AIDS Cohort Study, 1990-1998. Neurology. 2001;56(2):257–260. doi: 10.1212/wnl.56.2.257. [DOI] [PubMed] [Google Scholar]
- 123.Cysique LA, Brew BJ. Prevalence of non-confounded HIV-associated neurocognitive impairment in the context of plasma HIV RNA suppression. J. Neurovirol. 2011;17(2):176–183. doi: 10.1007/s13365-011-0021-x. [DOI] [PubMed] [Google Scholar]
- 124.Cysique LA, Maruff P, Bain MP, Wright E, Brew BJ. HIV and age do not substantially interact in HIV-associated neurocognitive impairment. J. Neuropsychiatry Clin. Neurosci. 2011;23(1):83–89. doi: 10.1176/jnp.23.1.jnp83. [DOI] [PubMed] [Google Scholar]
- 125.Wendelken LA, Valcour V. Impact of HIV and aging on neuropsychological function. J. Neurovirol. 2012;18(4):256–263. doi: 10.1007/s13365-012-0094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126■.Morgan EE, Woods SP, Delano-Wood L, Bondi MW, Grant I. Intraindividual variability in HIV infection: evidence for greater neurocognitive dispersion in older HIV seropositive adults. Neuropsychology. 2011;25(5):645–654. doi: 10.1037/a0023792. Demonstrated that older HIV-1-infected patients had a greater range of function across a range of tests, which could reflect cognitive dyscontrol, potentially driven by the effects of aging and HIV-1 infection on the prefrontostriatal systems.
- 127.Hardy DJ, Hinkin CH, Satz P, Stenquist PK, van Gorp WG, Moore LH. Age differences and neurocognitive performance in HIV-infected adults. NZ J. Psychol. 1999;28(2):94–101. [Google Scholar]
- 128.Kissel EC, Pukay-Martin ND, Bornstein RA. The relationship between age and cognitive function in HIV-infected men. J. Neuropsychiatry Clin. Neurosci. 2005;17(2):180–184. doi: 10.1176/jnp.17.2.180. [DOI] [PubMed] [Google Scholar]
- 129.Valcour V, Paul R, Neuhaus J, Shikuma C. The effects of age and HIV on neuropsychological performance. J. Int. Neuropsychol. Soc. 2010;17(1):190–195. doi: 10.1017/S1355617710001438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Schweinsburg BC, Taylor MJ, Alhassoon OM, et al. Brain mitochondrial injury in human immunodeficiency virus-seropositive (HIV+) individuals taking nucleoside reverse transcriptase inhibitors. J. Neurovirol. 2005;11(4):356–364. doi: 10.1080/13550280591002342. [DOI] [PubMed] [Google Scholar]
- 131.Stern Y. Cognitive reserve. Neuropsychologia. 2009;47(10):2015–2028. doi: 10.1016/j.neuropsychologia.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Nakamoto BK, Valcour VG, Kallianpur K, et al. Impact of cerebrovascular disease on cognitive function in HIV-infected patients. J. Acquir. Immune Defic. Syndr. 2011;57(3):e66–e68. doi: 10.1097/QAI.0b013e31821ff8bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Nakamoto B, Jahanshad N, Kallianpur K, Shikuma C, Valcour V, Thompson P. Impact of ApoE and cerebrovascular risk factors on brain structure and cognition in HIV in the HAART era; Presented at: 17th Conference on Retrovirueses and Opportunistic Infections; San Francisco, CA, USA. 2010.Feb 16–19, [Google Scholar]
- 134.Nakamoto BK, Jahanshad N, McMurtray A, et al. Cerebrovascular risk factors and brain microstructural abnormalities on diffusion tensor images in HIV-infected individuals. J. Neurovirol. 2012;18(4):303–312. doi: 10.1007/s13365-012-0106-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Langford SE, Ananworanich J, Cooper DA. Predictors of disease progression in HIV infection: a review. AIDS Res. Ther. 2007;4:11. doi: 10.1186/1742-6405-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Nguyen N, Holodniy M. HIV infection in the elderly. Clin. Interv. Aging. 2008;3(3):453–472. doi: 10.2147/cia.s2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Panel on Antiretroviral Guidelines for Adults and Adolescents . Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents. Department of Health and Human Services; Washington, DC, USA: 2011. [Google Scholar]
- 138.Bhatia R, Narain JP. Guidelines for HIV Diagnosis and Monitoring of Antiretroviral Therapy – South East Asia Regional Branch. WHO Press; Switzerland: 2005. [Google Scholar]
- 139.Lyles RH, Munoz A, Yamashita TE, et al. Natural history of human immunodeficiency virus type 1 viremia after seroconversion and proximal to AIDS in a large cohort of homosexual men. Multicenter AIDS Cohort Study. J. Infect. Dis. 2000;181(3):872–880. doi: 10.1086/315339. [DOI] [PubMed] [Google Scholar]
- 140.Thiebaut R, Pellegrin I, Chene G, et al. Immunological markers after long-term treatment interruption in chronically HIV-1 infected patients with CD4 cell count above 400 × 106 cells/l. AIDS. 2005;19(1):53–61. doi: 10.1097/00002030-200501030-00006. [DOI] [PubMed] [Google Scholar]
- 141.Arnaout RA, Lloyd AL, O’Brien TR, Goedert JJ, Leonard JM, Nowak MA. A simple relationship between viral load and survival time in HIV-1 infection. Proc. Natl Acad. Sci. USA. 1999;96(20):11549–11553. doi: 10.1073/pnas.96.20.11549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.O’Brien TR, Blattner WA, Waters D, et al. Serum HIV-1 RNA levels and time to development of AIDS in the Multicenter Hemophilia Cohort Study. JAMA. 1996;276(2):105–110. [PubMed] [Google Scholar]
- 143.Ferro S, Salit IE. HIV infection in patients over 55 years of age. J. Acquir. Immune Defic. Syndr. 1992;5(4):348–353. [PubMed] [Google Scholar]
- 144.Ena J, Valls V, Lopez Aldeguer J, et al. Clinical presentation of HIV infection in patients aged 50 years or older. J. Infect. 1999;37(3):213–216. doi: 10.1016/s0163-4453(98)91828-x. [DOI] [PubMed] [Google Scholar]
- 145.Cuzin L, Delpierre C, Gerard S, Massip P, Marchou B. Immunologic and clinical responses to highly active antiretroviral therapy in patients with HIV infection aged >50 years. Clin. Infect. Dis. 2007;45(5):654–657. doi: 10.1086/520652. [DOI] [PubMed] [Google Scholar]
- 146■.Greenbaum AH, Wilson LE, Keruly JC, Moore RD, Gebo KA. Effect of age and HAART regimen on clinical response in an urban cohort of HIV-infected individuals. AIDS. 2008;22(17):2331–2339. doi: 10.1097/QAD.0b013e32831883f9. Time to virologic suppression after the initiation of HAART was shorter in older versus younger patients, and there was no difference in CD4+ T-cell count.
- 147.Kalayjian RC, Spritzler J, Pu M, et al. mechanisms of T cell reconstitution can be identified by estimating thymic volume in adult HIV-1 disease. J. Infect. Dis. 2005;192(9):1577–1587. doi: 10.1086/466527. [DOI] [PubMed] [Google Scholar]
- 148.Manfredi R, Chiodo F. A case-control study of virological and immunological effects of highly active antiretroviral therapy in HIV-infected patients with advanced age. AIDS. 2000;14(10):1475–1477. doi: 10.1097/00002030-200007070-00034. [DOI] [PubMed] [Google Scholar]
- 149■■.Nogueras M, Navarro G, Anton E, et al. Epidemiological and clinical features, response to HAART, and survival in HIV-infected patients diagnosed at the age of 50 or more. BMC Infect. Dis. 2006;6:159. doi: 10.1186/1471-2334-6-159. Prospective cohort study that demonstrated that older HIV-1-infected patients have different epidemiological features. Although the immunologic and virologic responses to therapy were good, older patients did not achieve the same CD4+ T-cell counts as younger patients, probably because they had lower counts at their first clinic visit.
- 150.Orlando G, Meraviglia P, Cordier L, et al. Antiretroviral treatment and age-related comorbidities in a cohort of older HIV-infected patients. HIV Med. 2006;7(8):549–557. doi: 10.1111/j.1468-1293.2006.00420.x. [DOI] [PubMed] [Google Scholar]
- 151.Tumbarello M, Rabagliati R, De Gaetano Donati K, et al. Older age does not influence CD4 cell recovery in HIV-1 infected patients receiving highly active antiretroviral therapy. BMC Infect. Dis. 2004;4:46. doi: 10.1186/1471-2334-4-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Tumbarello M, Rabagliati R, De Gaetano Donati K, et al. Older HIV-positive patients in the era of highly active antiretroviral therapy: changing of a scenario. AIDS. 2002;17(1):128–131. doi: 10.1097/00002030-200301030-00020. [DOI] [PubMed] [Google Scholar]
- 153.Yamashita TE, Phair JP, Munoz A, et al. Immunologic and virologic response to highly active antiretroviral therapy in the Multicenter AIDS Cohort Study. AIDS. 2001;15(6):735–746. doi: 10.1097/00002030-200104130-00009. [DOI] [PubMed] [Google Scholar]
- 154.Manfredi R, Calza L, Cocchi D, Chiodo F. Antiretroviral treatment and advanced age: epidemiologic, laboratory, and clinical features in the elderly. J. Acquir. Immune Defic. Syndr. 2003;33(1):112–114. doi: 10.1097/00126334-200305010-00016. [DOI] [PubMed] [Google Scholar]
- 155.Grimes RM, Otiniano ME, Rodriguez-Barradas MC, Lai D. Clinical experience with human immunodeficiency virus-infected older patients in the era of effective antiretroviral therapy. Clin. Infect. Dis. 2002;34(11):1530–1533. doi: 10.1086/340404. [DOI] [PubMed] [Google Scholar]
- 156.Goodkin K, Shapshak P, Asthana D, et al. Older age and plasma viral load in HIV-1 infection. AIDS. 2004;18(Suppl. 1):S87–S98. [PubMed] [Google Scholar]
- 157■.Grabar S, Kousignian I, Sobel A, et al. Immunologic and clinical responses to highly active antiretroviral therapy over 50 years of age. Results from the French Hospital Database on HIV. AIDS. 2004;18(15):2029–2038. doi: 10.1097/00002030-200410210-00007. Compared older and younger patients and demonstrated that older patients had an immune response to HAART with a better virologic response than the younger group; however, CD4+ T-cell reconstitution was slower, suggesting a higher risk of clinical progression.
- 158.Knobel H, Guelar A, Valldecillo G, et al. Response to highly active antiretroviral therapy in HIV-infected patients aged 60 years or older after 24 months follow-up. AIDS. 2001;15(12):1591–1593. doi: 10.1097/00002030-200108170-00025. [DOI] [PubMed] [Google Scholar]
- 159.Navarro G, Nogueras MM, Segura F, et al. HIV-1 infected patients older than 50 years. PISCIS cohort study. J. Infect. 2008;57(1):64–71. doi: 10.1016/j.jinf.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 160.Perez JL, Moore RD. Greater effect of highly active antiretroviral therapy on survival in people aged > or =50 years compared with younger people in an urban observational cohort. Clin. Infect. Dis. 2003;36(2):212–218. doi: 10.1086/345669. [DOI] [PubMed] [Google Scholar]
- 161.Silverberg MJ, Leyden W, Horberg MA, DeLorenze GN, Klein D, Quesenberry CP., Jr. Older age and the response to and tolerability of antiretroviral therapy. Arch. Intern. Med. 2007;167(7):684–691. doi: 10.1001/archinte.167.7.684. [DOI] [PubMed] [Google Scholar]
- 162.Vallecillo G, Knobel H, Guelar A, Saballs P. Evolution of a cohort of patients over 60 years old with HIV infection treated with highly-active antiretroviral therapy. Enferm. Infecc. Microbiol. Clin. 2005;23(6):390–391. doi: 10.1157/13076185. [DOI] [PubMed] [Google Scholar]
- 163.Paredes R, Mocroft A, Kirk O, et al. Predictors of virological success and ensuing failure in HIV-positive patients starting highly active antiretroviral therapy in Europe: results from the EuroSIDA study. Arch. Intern. Med. 2000;160(8):1123–1132. doi: 10.1001/archinte.160.8.1123. [DOI] [PubMed] [Google Scholar]
- 164.Wellons MF, Sanders L, Edwards LJ, Bartlett JA, Heald AE, Schmader KE. HIV infection: treatment outcomes in older and younger adults. J. Am. Geriatr. Soc. 2002;50(4):603–607. doi: 10.1046/j.1532-5415.2002.50152.x. [DOI] [PubMed] [Google Scholar]
- 165.Carrieri MP, Leport C, Protopopescu C, et al. Factors associated with nonadherence to highly active antiretroviral therapy: a 5-year follow-up analysis with correction for the bias induced by missing data in the treatment maintenance phase. J. Acquir. Immune Defic. Syndr. 2006;41(4):477–485. doi: 10.1097/01.qai.0000186364.27587.0e. [DOI] [PubMed] [Google Scholar]
- 166.Barclay TR, Hinkin CH, Castellon SA, et al. Age-associated predictors of medication adherence in HIV-positive adults: health beliefs, self-efficacy, and neurocognitive status. Health Psychol. 2007;26(1):40–49. doi: 10.1037/0278-6133.26.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Chinen J, Shearer WT. Molecular virology and immunology of HIV infection. J. Allergy Clin. Immunol. 2002;110(2):189–198. doi: 10.1067/mai.2002.126226. [DOI] [PubMed] [Google Scholar]
- 168.Zhou J, Kumarasamy N. Predicting short-term disease progression among HIV-infected patients in Asia and the Pacific region: preliminary results from the TREAT Asia HIV Observational Database (TAHOD) HIV Med. 2005;6(3):216–223. doi: 10.1111/j.1468-1293.2005.00292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Phillips AN, Lundgren JD. The CD4 lymphocyte count and risk of clinical progression. Curr. Opin. HIV AIDS. 2006;1(1):43–49. doi: 10.1097/01.COH.0000194106.12816.b1. [DOI] [PubMed] [Google Scholar]
- 170.Phillips A, Pezzotti P. Short-term risk of AIDS according to current CD4 cell count and viral load in antiretroviral drug-naive individuals and those treated in the monotherapy era. AIDS. 2004;18(1):51–58. doi: 10.1097/00002030-200401020-00006. [DOI] [PubMed] [Google Scholar]
- 171■.Hogg RS, Yip B, Chan KJ, et al. Rates of disease progression by baseline CD4 cell count and viral load after initiating triple-drug therapy. JAMA. 2001;286(20):2568–2577. doi: 10.1001/jama.286.20.2568. Demonstrated that for patients initiating HAART with CD4+ T-cell counts of at least 200 cells/mm3, progression to AIDS and death was at a uniformly low rate. The highest levels of disease progression and death clustered around those who started therapy with CD4+ T-cell counts of less than 200 cells/mm3.
- 172.Goujard C, Bonarek M, Meyer L, et al. CD4 cell count and HIV DNA level are independent predictors of disease progression after primary HIV type 1 infection in untreated patients. Clin. Infect. Dis. 2006;42(5):709–715. doi: 10.1086/500213. [DOI] [PubMed] [Google Scholar]
- 173.de Wolf F, Spijkerman I, Schellekens PT, et al. AIDS prognosis based on HIV-1 RNA, CD4+ T-cell count and function: markers with reciprocal predictive value over time after seroconversion. AIDS. 1997;11(15):1799–1806. doi: 10.1097/00002030-199715000-00003. [DOI] [PubMed] [Google Scholar]
- 174.Cozzi Lepri A, Katzenstein TL, Ullum H, et al. The relative prognostic value of plasma HIV RNA levels and CD4 lymphocyte counts in advanced HIV infection. AIDS. 1998;12(13):1639–1643. doi: 10.1097/00002030-199813000-00011. [DOI] [PubMed] [Google Scholar]
- 175.Moore RD, Chaisson RE. Natural history of HIV infection in the era of combination antiretroviral therapy. AIDS. 1999;13(14):1933–1942. doi: 10.1097/00002030-199910010-00017. [DOI] [PubMed] [Google Scholar]
- 176.Avelino-Silva VI, Ho YL, Avelino-Silva TJ, Santos Sde S. Aging and HIV infection. Ageing Res. Rev. 2010;10(1):163–172. doi: 10.1016/j.arr.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 177.Blanco JR, Caro AM, Perez-Cachafeiro S, et al. HIV infection and aging. AIDS Rev. 2010;12(4):218–230. [PubMed] [Google Scholar]
- 178.Shah S, Mildvan D. HIV and aging. Curr. Infect. Dis. Rep. 2006;8(3):241–247. doi: 10.1007/s11908-006-0065-x. [DOI] [PubMed] [Google Scholar]
- 179.Douek DC, McFarland RD, Keiser PH, et al. Changes in thymic function with age and during the treatment of HIV infection. Nature. 1999;396(6712):690–695. doi: 10.1038/25374. [DOI] [PubMed] [Google Scholar]
- 180.Casau NC. Perspective on HIV infection and aging: emerging research on the horizon. Clin. Infect. Dis. 2005;41(6):855–863. doi: 10.1086/432797. [DOI] [PubMed] [Google Scholar]
- 181.Douek DC, Picker LJ, Koup RA. T cell dynamics in HIV-1 infection. Annu. Rev. Immunol. 2003;21:265–304. doi: 10.1146/annurev.immunol.21.120601.141053. [DOI] [PubMed] [Google Scholar]
- 182.Effros RB, Allsopp R, Chiu CP, et al. Shortened telomeres in the expanded CD28− CD8+ cell subset in HIV disease implicate replicative senescence in HIV pathogenesis. AIDS. 1996;10(8):F17–F22. doi: 10.1097/00002030-199607000-00001. [DOI] [PubMed] [Google Scholar]
- 183■.Haynes L, Eaton SM, Burns EM, Randall TD, Swain SL. CD4 T cell memory derived from young naive cells functions well into old age, but memory generated from aged naive cells functions poorly. Proc. Natl Acad. Sci. USA. 2003;100(25):15053–15058. doi: 10.1073/pnas.2433717100. Examined the ability of naive CD4+ T cells from young and old transgenic mice to establish functional memory. Memory CD4+ T cells from older mice responded poorly both ex vivo and in vivo.
- 184.Haynes L, Eaton SM, Burns EM, Randall TD, Swain SL. Newly generated CD4 T cells in aged animals do not exhibit age-related defects in response to antigen. J. Exp. Med. 2005;201(6):845–851. doi: 10.1084/jem.20041933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Miller RA, Garcia G, Kirk CJ, Witkowski JM. Early activation defects in T lymphocytes from aged mice. Immunol. Rev. 1998;160:79–90. doi: 10.1111/j.1600-065x.1997.tb01029.x. [DOI] [PubMed] [Google Scholar]
- 186.Kapasi ZF, Murali-Krishna K, McRae ML, Ahmed R. Defective generation but normal maintenance of memory T cells in old mice. Eur. J. Immunol. 2002;32(6):1567–1573. doi: 10.1002/1521-4141(200206)32:6<1567::AID-IMMU1567>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 187.Phillips AN, Lee CA, Elford J, et al. More rapid progression to AIDS in older HIV-infected people: the role of CD4+ T-cell counts. J. Acquir. Immune Defic. Syndr. 1991;4(10):970–975. [PubMed] [Google Scholar]
- 188.Operskalski EA, Stram DO, Lee H, et al. Transfusion Safety Study Group Human immunodeficiency virus type 1 infection: relationship of risk group and age to rate of progression to AIDS. J. Infect. Dis. 1995;172(3):648–655. doi: 10.1093/infdis/172.3.648. [DOI] [PubMed] [Google Scholar]
- 189.Viard JP, Mocroft A, Chiesi A, et al. Influence of age on CD4 cell recovery in human immunodeficiency virus-infected patients receiving highly active antiretroviral therapy: evidence from the EuroSIDA study. J. Infect. Dis. 2001;183(8):1290–1294. doi: 10.1086/319678. [DOI] [PubMed] [Google Scholar]
- 190.Lederman MM, McKinnis R, Kelleher D, et al. Cellular restoration in HIV infected persons treated with abacavir and a protease inhibitor: age inversely predicts naive CD4 cell count increase. AIDS. 2000;14(17):2635–2642. doi: 10.1097/00002030-200012010-00002. [DOI] [PubMed] [Google Scholar]
- 191.Cohen Stuart J, Hamann D, Borleffs J, et al. Reconstitution of naive T cells during antiretroviral treatment of HIV-infected adults is dependent on age. AIDS. 2002;16(17):2263–2266. doi: 10.1097/00002030-200211220-00005. [DOI] [PubMed] [Google Scholar]
- 192.Florence E, Lundgren J, Dreezen C, et al. Factors associated with a reduced CD4 lymphocyte count response to HAART despite full viral suppression in the EuroSIDA study. HIV Med. 2003;4(3):255–262. doi: 10.1046/j.1468-1293.2003.00156.x. [DOI] [PubMed] [Google Scholar]
- 193.Kaufmann GR, Furrer H, Ledergerber B, et al. Characteristics, determinants, and clinical relevance of CD4 T cell recovery to <500 cells/microL in HIV type 1-infected individuals receiving potent antiretroviral therapy. Clin. Infect. Dis. 2005;41(3):361–372. doi: 10.1086/431484. [DOI] [PubMed] [Google Scholar]
- 194■■.Baker JV, Peng G, Rapkin J, et al. Poor initial CD4+ recovery with antiretroviral therapy prolongs immune depletion and increases risk for AIDS and non-AIDS diseases. J. Acquir. Immune Defic. Syndr. 2008;48(5):541–546. doi: 10.1097/QAI.0b013e31817bebb3. Determined that older age and lower screening HIV RNA, but not screening CD4+ T-cell counts, were associated with a poor CD4+ T-cell recovery. This suggested that impaired immune recovery, despite effective HAART, resulted in a longer time spent at lower CD4+ T-cell counts.
- 195.Goetz MB, Boscardin WJ, Wiley D, Alkasspooles S. Decreased recovery of CD4 lymphocytes in older HIV-infected patients beginning highly active antiretroviral therapy. AIDS. 2001;15(12):1576–1579. doi: 10.1097/00002030-200108170-00017. [DOI] [PubMed] [Google Scholar]
- 196.Szadkowski L, Tseng A, Walmsley SL, Salit I, Raboud JM. Effects of age on virologic suppression and CD4 cell response in HIV-positive patients initiating combination antiretroviral therapy. AIDS Res. Hum. Retroviruses. 2012;28(12):1579–1583. doi: 10.1089/AID.2012.0018. [DOI] [PubMed] [Google Scholar]
- 197.Branas F, Berenguer J, Sanchez-Conde M, et al. The eldest of older adults living with HIV: response and adherence to highly active antiretroviral therapy. Am. J. Med. 2008;121(9):820–824. doi: 10.1016/j.amjmed.2008.05.027. [DOI] [PubMed] [Google Scholar]
- 198■.Hearps AC, Angelovich TA, Jaworowski A, Mills J, Landay AL, Crowe SM. HIV infection and aging of the innate immune system. Sexual Health. 2011;8(4):453–464. doi: 10.1071/SH11028. Review focusing on the impact of HIV-1 infection on the function and aging of innate immune cells. Discusses potential drivers behind premature immunosenescence.
- 199.Seidler S, Zimmermann HW, Bartneck M, Trautwein C, Tacke F. Age-dependent alterations of monocyte subsets and monocyte-related chemokine pathways in healthy adults. BMC Immunol. 2010;11:30. doi: 10.1186/1471-2172-11-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200■.Nyugen J, Agrawal S, Gollapudi S, Gupta S. Impaired functions of peripheral blood monocyte subpopulations in aged humans. J. Clin. Immunol. 2010;30(6):806–813. doi: 10.1007/s10875-010-9448-8. Identified and compared four subpopulations of monocytes from the peripheral blood of young and elderly patients, demonstrating that in aging, functions are differentially impaired with respect to cytokine production, Toll-like receptor expression and ERK–MAPK signaling.
- 201.Ancuta P, Kamat A, Kunstman KJ, et al. Microbial translocation is associated with increased monocyte activation and dementia in AIDS patients. PLoS One. 2008;3(6):e2516. doi: 10.1371/journal.pone.0002516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Thieblemont N, Weiss L, Sadeghi HM, Estcourt C, Haeffner-Cavaillon N. CD14lowCD16high: a cytokine-producing monocyte subset which expands during human immunodeficiency virus infection. Eur. J. Immunol. 1995;25(12):3418–3424. doi: 10.1002/eji.1830251232. [DOI] [PubMed] [Google Scholar]
- 203.Burdo TH, Lentz MR, Autissier P, et al. Soluble CD163 made by monocyte/macrophages is a novel marker of HIV activity in early and chronic infection prior to and after anti-retroviral therapy. J. Infect. Dis. 2011;204(1):154–163. doi: 10.1093/infdis/jir214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Burdo TH, Lo J, Abbara S, et al. Soluble CD163, a novel marker of activated macrophages, is elevated and associated with noncalcified coronary plaque in HIV-infected patients. J. Infect. Dis. 2011;204(8):1227–1236. doi: 10.1093/infdis/jir520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Williams K, Burdo TH. Monocyte mobilization, activation markers, and unique macrophage populations in the brain: observations from SIV infected monkeys are informative with regard to pathogenic mechanisms of HIV infection in humans. J. Neuroimmune Pharmacol. 2011;7(2):363–371. doi: 10.1007/s11481-011-9330-3. [DOI] [PubMed] [Google Scholar]
- 206.Zanni MV, Burdo TH, Makimura H, Williams KC, Grinspoon SK. Relationship between monocyte/macrophage activation marker soluble CD163 and insulin resistance in obese and normal-weight subjects. Clin. Endocrinol. 2011;77(3):385–390. doi: 10.1111/j.1365-2265.2011.04284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Villanueva JL, Solana R, Alonso MC, Pena J. Changes in the expression of HLA-class II antigens on peripheral blood monocytes from aged humans. Disease Markers. 1990;8(2):85–91. [PubMed] [Google Scholar]
- 208.Plackett TP, Boehmer ED, Faunce DE, Kovacs EJ. Aging and innate immune cells. J. Leukoc. Biol. 2004;76(2):291–299. doi: 10.1189/jlb.1103592. [DOI] [PubMed] [Google Scholar]
- 209.Gomez CR, Boehmer ED, Kovacs EJ. The aging innate immune system. Curr. Opin. Immunol. 2005;17(5):457–462. doi: 10.1016/j.coi.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 210.Butcher SK, Chahal H, Nayak L, et al. Senescence in innate immune responses: reduced neutrophil phagocytic capacity and CD16 expression in elderly humans. J. Leukoc. Biol. 2001;70(6):881–886. [PubMed] [Google Scholar]
- 211.Torre D, Gennero L, Baccino FM, Speranza F, Biondi G, Pugliese A. Impaired macrophage phagocytosis of apoptotic neutrophils in patients with human immunodeficiency virus type 1 infection. Clin. Diagn. Lab. Immunol. 2002;9(5):983–986. doi: 10.1128/CDLI.9.5.983-986.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212■.Noursadeghi M, Katz DR, Miller RF. HIV-1 infection of mononuclear phagocytic cells: the case for bacterial innate immune deficiency in AIDS. Lancet Infect. Dis. 2006;6(12):794–804. doi: 10.1016/S1473-3099(06)70656-9. Discusses the effects of HIV-1 on bacterial innate immunity. Also discusses the mechanisms for HIV-1-mediated disruption of the innate immune response.
- 213.Jenny NS, Tracy RP, Ogg MS, et al. In the elderly, interleukin-6 plasma levels and the −174G>C polymorphism are associated with the development of cardiovascular disease. Arterioscler. Thromb. Vasc. Biol. 2002;22(12):2066–2071. doi: 10.1161/01.atv.0000040224.49362.60. [DOI] [PubMed] [Google Scholar]
- 214■■.Breen EC, Rezai AR, Nakajima K, et al. Infection with HIV is associated with elevated IL-6 levels and production. J. Immunol. 1990;144(2):480–484. Demonstrates an association between elevated IL-6 and HIV-1 infection, and suggests that the IL-6 overproduction may contribute to the polyclonal B-cell activation seen during HIV-1 infection.
- 215.Hasegawa Y, Sawada M, Ozaki N, Inagaki T, Suzumura A. Increased soluble tumor necrosis factor receptor levels in the serum of elderly people. Gerontology. 2000;46(4):185–188. doi: 10.1159/000022157. [DOI] [PubMed] [Google Scholar]
- 216.Shurin GV, Yurkovetsky ZR, Chatta GS, Tourkova IL, Shurin MR, Lokshin AE. Dynamic alteration of soluble serum biomarkers in healthy aging. Cytokine. 2007;39(2):123–129. doi: 10.1016/j.cyto.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 217.Godfried MH, van der Poll T, Jansen J, et al. Soluble receptors for tumour necrosis factor: a putative marker of disease progression in HIV infection. AIDS. 1993;7(1):33–36. [PubMed] [Google Scholar]
- 218.Zangerle R, Fuchs D, Sarcletti M, et al. Increased concentrations of soluble tumor necrosis factor receptor 75 but not of soluble intercellular adhesion molecule-1 are associated with the decline of CD4+ lymphocytes in HIV infection. Clin. Immunol. Immunopathol. 1994;72(3):328–334. doi: 10.1006/clin.1994.1149. [DOI] [PubMed] [Google Scholar]
- 219.French MA, King MS, Tschampa JM, da Silva BA, Landay AL. Serum immune activation markers are persistently increased in patients with HIV infection after 6 years of antiretroviral therapy despite suppression of viral replication and reconstitution of CD4+ T cells. J. Infect. Dis. 2009;200(8):1212–1215. doi: 10.1086/605890. [DOI] [PubMed] [Google Scholar]
- 220.Gunter TE, Sheu SS. Characteristics and possible functions of mitochondrial Ca2+ transport mechanisms. Biochim. Biophys. Acta. 2009;1787(11):1291–1308. doi: 10.1016/j.bbabio.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Chan DC. Mitochondrial fusion and fission in mammals. Annu. Rev. Cell Dev. Biol. 2006;22:79–99. doi: 10.1146/annurev.cellbio.22.010305.104638. [DOI] [PubMed] [Google Scholar]
- 222.Anderson S, Bankier AT, Barrell BG, et al. Sequence and organization of the human mitochondrial genome. Nature. 1981;290(5806):457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- 223.Shmookler Reis RJ, Goldstein S. Mitochondrial DNA in mortal and immortal human cells. Genome number, integrity, and methylation. J. Biol. Chem. 1983;258(15):9078–9085. [PubMed] [Google Scholar]
- 224.Lodish HF. Molecular Cell Biology. 4th Edition WH Freeman; NY, USA: 2000. [Google Scholar]
- 225.Perry SW, Norman JP, Barbieri J, Brown EB, Gelbard HA. Mitochondrial membrane potential probes and the proton gradient: a practical usage guide. Biotechniques. 2011;50(2):98–115. doi: 10.2144/000113610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Pfanner N, Truscott KN. Powering mitochondrial protein import. Nat. Struct. Biol. 2002;9(4):234–236. doi: 10.1038/nsb0402-234. [DOI] [PubMed] [Google Scholar]
- 227.Petronilli V, Cola C, Massari S, Colonna R, Bernardi P. Physiological effectors modify voltage sensing by the cyclosporin A-sensitive permeability transition pore of mitochondria. J. Biol. Chem. 1993;268(29):21939–21945. [PubMed] [Google Scholar]
- 228.Rasola A, Bernardi P. Mitochondrial permeability transition in Ca2+-dependent apoptosis and necrosis. Cell Calcium. 2011;50(3):222–233. doi: 10.1016/j.ceca.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 229.Apostolova N, Blas-Garcia A, Esplugues JV. Mitochondrial interference by anti-HIV drugs: mechanisms beyond Pol-gamma inhibition. Trends Pharmacol. Sci. 2011;32(12):715–725. doi: 10.1016/j.tips.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 230.Larsson NG. Somatic mitochondrial DNA mutations in mammalian aging. Annu. Rev. Biochem. 2010;79:683–706. doi: 10.1146/annurev-biochem-060408-093701. [DOI] [PubMed] [Google Scholar]
- 231.Castro Mdel R, Suarez E, Kraiselburd E, et al. Aging increases mitochondrial DNA damage and oxidative stress in liver of rhesus monkeys. Exp. Gerontol. 2011;47(1):29–37. doi: 10.1016/j.exger.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232■.Trifunovic A, Wredenberg A, Falkenberg M, et al. Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature. 2004;429(6990):417–423. doi: 10.1038/nature02517. Demonstrates that knock-in mice develop a mitochondrial DNA (mtDNA) mutator phenotype with an increase in the levels of point mutations, as well as increased amounts of deleted mtDNA. This increase in somatic mtDNA mutations is associated with reduced lifespan and premature onset of aging-related phenotypes.
- 233■■.Johnson AA, Ray AS, Hanes J, et al. Toxicity of antiviral nucleoside analogs and the human mitochondrial DNA polymerase. J. Biol. Chem. 2001;276(44):40847–40857. doi: 10.1074/jbc.M106743200. Examines the role of mitochondrial polymerase in the toxicity of nucleoside reverse transcriptase inhibitors (NRTIs). Also examines the rate of exonuclease removal of each analog after incorporation.
- 234.Brinkman K, ter Hofstede HJ, Burger DM, Smeitink JA. Koopmans PP. Adverse effects of reverse transcriptase inhibitors: mitochondrial toxicity as common pathway. AIDS. 1998;12(14):1735–1744. doi: 10.1097/00002030-199814000-00004. [DOI] [PubMed] [Google Scholar]
- 235.Curran A, Ribera E. From old to new nucleoside reverse transcriptase inhibitors: changes in body fat composition, metabolic parameters and mitochondrial toxicity after the switch from thymidine analogs to tenofovir or abacavir. Expert Opin. Drug Saf. 2011;10(3):389–406. doi: 10.1517/14740338.2011.542145. [DOI] [PubMed] [Google Scholar]
- 236■.Chiappini F, Teicher E, Saffroy R, Debuire B, Vittecoq D, Lemoine A. Relationship between polymerase gamma (POLG) polymorphisms and antiretroviral therapy-induced lipodystrophy in HIV-1 infected patients: a case-control study. Curr. HIV Res. 2009;7(2):244–253. doi: 10.2174/157016209787581409. Explores the relationship between selected polymorphisms of polymerase-γ and lipodystrophy related to NRTIs. Demonstrated a link between polymorphisms in E1143 and lipodystrophy, which was associated with a significant decrease of mtDNA in peripheral blood mononuclear cells and with stavudine therapy.
- 237.Hulgan T, Haubrich R, Riddler SA, et al. European mitochondrial DNA haplogroups and metabolic changes during antiretroviral therapy in AIDS Clinical Trials Group study A5142. AIDS. 2010;25(1):37–47. doi: 10.1097/QAD.0b013e32833f9d02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Nasi M, Guaraldi G, Orlando G, et al. Mitochondrial DNA haplogroups and highly active antiretroviral therapy-related lipodystrophy. Clin. Infect. Dis. 2008;47(7):962–968. doi: 10.1086/591706. [DOI] [PubMed] [Google Scholar]
- 239.Soong NW, Hinton DR, Cortopassi G, Arnheim N. Mosaicism for a specific somatic mitochondrial DNA mutation in adult human brain. Nat. Genet. 1992;2(4):318–323. doi: 10.1038/ng1292-318. [DOI] [PubMed] [Google Scholar]
- 240.Corral-Debrinski M, Horton T, Lott MT, Shoffner JM, Beal MF, Wallace DC. Mitochondrial DNA deletions in human brain: regional variability and increase with advanced age. Nat. Genet. 1992;2(4):324–329. doi: 10.1038/ng1292-324. [DOI] [PubMed] [Google Scholar]
- 241■■.Payne BA, Wilson IJ, Hateley CA, et al. Mitochondrial aging is accelerated by antiretroviral therapy through the clonal expansion of mtDNA mutations. Nat. Genet. 2011;43(8):806–810. doi: 10.1038/ng.863. Demonstrates that patients treated with common NRTIs accumulate somatic mtDNA mutations mirroring those seen much later in normal aging.
- 242.Feldman MD. Sex, AIDS, and the elderly. Arch. Intern. Med. 1994;154(1):19–20. [PubMed] [Google Scholar]
- 243.Schable B, Chu SY, Diaz T. Characteristics of women 50 years of age or older with heterosexually acquired AIDS. Am. J. Public Health. 1996;86(11):1616–1618. doi: 10.2105/ajph.86.11.1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Stall R, Catania J. AIDS risk behaviors among late middle-aged and elderly Americans. The National AIDS Behavioral Surveys. Arch. Intern. Med. 1994;154(1):57–63. [PubMed] [Google Scholar]
- 245.Gerbert B, Maguire BT, Coates TJ. Are patients talking to their physicians about AIDS? Am. J. Public Health. 1990;80(4):467–469. doi: 10.2105/ajph.80.4.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.el-Sadr W, Gettler J. Unrecognized human immunodeficiency virus infection in the elderly. Arch. Intern. Med. 1995;155(2):184–186. [PubMed] [Google Scholar]
- 247.Bangen KJ, Beiser A, Delano-Wood L, et al. APOE genotype modifies the relationship between midlife vascular risk factors and later cognitive decline; Presented at: 41st Annual Meeting of the International Neuropsychological Society; Waikoloa, HI, USA. 2013; Feb 6–9, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Maagaard A, Kvale D. Mitochondrial toxicity in HIV-infected patients both off and on antiretroviral treatment: a continuum or distinct underlying mechanisms? J. Antimicrob. Chemother. 2009;64(5):901–909. doi: 10.1093/jac/dkp316. [DOI] [PubMed] [Google Scholar]