Abstract
Reduced EphB4 expression is observed during vein graft adaptation and is associated with increased venous wall thickening. These findings suggest that EphB4 may mediate normal adult venous endothelial cell (EC) function and vein graft adaptation. We therefore tested the functional significance of EphB4 using EC with genetically reduced EphB4 signaling. EC were isolated from EphB4+/+ and EphB4+/− mice. In vitro function was assessed through EC proliferation, migration, nitric oxide (NO) synthesis, and chemokine production. A mouse vein graft model was used to correlate in vitro findings with in vivo vein grafts. Smooth muscle cells (SMC) were subjected to proliferation and migration assays using EphB4+/+ and EphB4+/− EC-conditioned medium. EphB4+/− EC exhibited diminished proliferation (P < 0.0001, n = 6), migration (P < 0.0001, n = 3), and NO production (P = 0.0012, n = 3). EphB4+/− EC had increased VEGF-A mRNA (P = 0.0006, n = 6) and protein (P = 0.0106, n = 3) as well as increased secretion of VEGF-A (P = 0.0010, n = 5), PDGF-BB (P < 0.0001, n = 6), and TGF-β1 (P < 0.0001, n = 6). EphB4+/−-conditioned medium promoted SMC proliferation (P < 0.0001, n = 7) and migration (P = 0.0358, n = 3). Vein grafts and EphB4+/− EC showed similarity with regard to VEGF-A and eNOS mRNA and protein expression. In conclusion, reduced venous EC EphB4 function is associated with a proangiogenic and mitogenic phenotype. EphB4+/− EC have increased secretion of SMC mitogens and reduced NO production that correlate with the thickened neointima formed during vein graft adaptation. These findings suggest that EphB4 remains active in adult venous EC and that loss of EphB4 plays a role in vein graft adaptation.
Keywords: EphB4, venous endothelial cells, vein graft remodeling
autogenous saphenous vein placed into arterial circulation as a bypass graft remains the gold standard of surgical treatment for critical limb ischemia. Reduced patency is routinely observed with the use of prosthetic graft alternatives, and it is generally believed that this inferior performance occurs secondary to the lack of an intact, functional, antithrombotic endothelium (23). Following arterialization, human vein grafts initially demonstrate outward remodeling, which in turn is followed by the progressive acquisition of wall stiffness (20) in a process that involves inflammation (21). Despite adaptive changes, vein graft failure secondary to progressive neointimal hyperplasia remains a major clinical problem. Currently, little is known about the molecular biology of vein graft remodeling especially regarding the balance between necessary adaptation and aberrant venous remodeling that manifests as pathological neointimal hyperplasia and vein graft failure (22).
The molecular distinction between arterial and venous endothelial cells (EC) is determined during embryonic development, and this distinction persists in the adult vasculature (27). EphB4, a receptor tyrosine kinase, is a marker of adult venous EC, whereas Ephrin-B2, membrane-bound ligand for EphB4, is predominantly expressed by arterial EC (1, 8, 27). However, it is not currently clear whether EphB4 plays a role in adult EC or whether it is simply a retained embryonic venous cellular identity marker (12). Analysis of patent human vein grafts has shown that placement of a vein into the arterial circulation results in the loss of EphB4 without an accompanying upregulation of arterial identity markers. This observation suggests that EphB4 may not just be a passive venous identity marker but rather an active mediator in adult EC homeostasis (12).
Furthermore, Muto et al. (18) have recently shown that EphB4 loss is associated with venous wall thickening and that stimulation of EphB4 signaling during vein graft adaptation facilitates both the preservation of venous identity as well as inhibition of wall thickening. These observations suggest that EphB4 is active in adult veins in vivo and that loss of EphB4 is critical for successful venous adaptation to the arterial environment. To test the functional significance of EphB4 activity in adult cells, we examined the effects of reduced EphB4 signaling on venous EC function in vitro and compared these changes to those that occur during vein graft adaptation in vivo.
MATERIALS AND METHODS
Antibodies and reagents.
Primary antibodies to the following antigens were obtained as follows: total-Akt, phospho-Akt, total-ERK1/2, phospho-ERK1/2, hsp90, VEGFR2, phosphotyrosine (Cell Signaling Technology, Danvers, MA); eNOS, phospho-eNOS, caveolin-1 (Cav-1; BD Biosciences, San Jose, CA), VEGF-A (Santa Cruz Biotechnology, Santa Cruz, CA), EphB4 (Abcam, Cambridge, MA); PCNA (Sigma-Aldrich, St. Louis, MO); BrdU Cell Proliferation Assay (Cell Signaling Technology); Dip Stain Kit (Volu-Sol, Salt Lake City, UT); and Ephrin-B2/Fc (R&D Systems, Minneapolis, MN).
Isolation of mouse lung endothelial cells.
All animal procedures were approved by Yale University's Institutional Animal Care and Use Committee and were performed in keeping with the National Institutes of Health ethical guidelines. Primary mouse lung EC were isolated as previously described (18). Briefly, under sterile conditions, lung tissue was collected from 6-wk-old EphB4+/+ and EphB4+/− mice (8), minced in 0.1% collagenase, and further homogenized by repeated passage through a 14-gauge needle. After 48 h, cells were immortalized by infection with a retrovirus expressing the polyoma middle T antigen. A second sortation was performed using magnetic beads conjugated with anti-mouse CD31; EC were collected using a magnetic sorter, plated, and passaged for experimental use.
Primary vascular smooth muscle cell isolation.
Primary mouse vascular smooth muscle cells (SMC) were isolated from the thoracic aortas of C57BL/6 mice using a combined collagenase and elastase digestion method (6). Isolated cells were then cultured and used for experiment immediately following one passage.
Animal vein graft model.
Fifteen-week-old C57BL/6 mice were used to generate in vivo matched vein and vein graft tissue as previously described (18). In brief, thoracic inferior vena cava (IVC) was harvested from donor mice and transplanted into the infrarenal aorta of recipient mice using reverse interposition grafting. A 10-0 continuous running suture was used to fashion the vein graft anastomosis. All implanted vein grafts were followed by ultrasound to ensure graft patency. Thoracic IVC (vein) and vein graft samples were harvested at 1 wk postoperatively for protein analysis.
Agarose gel electrophoresis of DNA.
Total DNA from EphB4+/+ and EphB4+/− EC was isolated and amplified using primers as previously published (8). DNA fragments were then separated out on a 5% agarose gel containing ethidium bromide, and a UV-light box was used for visualization.
ELISA assays.
EphB4+/+ and EphB4+/− EC were plated at equal cell densities, and cell-conditioned media were collected following a 24-h period of incubation. Analysis of the conditioned media was then performed using mouse VEGF-A, PDGF-BB, and TGF-β1 ELISA assays (R&D Systems). Samples were analyzed in duplicate form, and assays were used as directed by the manufacturer's product protocol.
BrdU SMC cell proliferation assay.
EphB4+/+ and EphB4+/− EC were plated at equal cell densities, and cell culture supernatants were collected following a 24-h period of incubation. SMC were then seeded at 3 × 104 cells/well in a 96-well plate and incubated with serum-free medium (SFM), 10% fetal bovine serum (FBS), or supernatant from EphB4+/+ and EphB4+/− EC. BrdU solution was prepared according to the manufacturer's protocol and added into cell culture medium. SMC were then incubated for 24 h. BrdU SMC incorporation was assayed according to product protocol, with plate absorbance read at 450 nm.
EC proliferating cell nuclear antigen (PCNA) proliferation assay.
EphB4+/+ and EphB4+/− EC were seeded at equal cell densities (1 × 104 cells/100 μl) and allowed to incubate overnight in FBS-containing culture medium. Following 24 h of incubation, cells were fixed, permeabilized, and incubated with anti-PCNA antibody according to manufacturer's protocol.
SMC migration assay.
Prior to beginning SMC migration, EphB4+/+ and EphB4+/− EC were plated at equal cell densities, and cell culture supernatants were collected following a 24-h period of incubation. SMC were serum starved for 15 h before the migration assay. SMC migration was assessed using 8-μm transwell inserts coated with type I collagen (Corning Life Science, Tewksbury, MA); SMC were placed in the transwell upper chambers in equal cell concentrations (1 × 105 cells/100 μl). SFM, 10% FBS, and conditioned medium from EphB4+/+ and EphB4+/− EC were used for SMC chemoattraction; chemoattractants were placed in equal volumes into the lower transwell chambers. After 8 h of incubation the cells were fixed and stained. Nonmigratory SMC on the upper side of the insert were scraped off with cotton swabs, and the SMC that migrated onto the lower side of the insert were then counted using light microscopy.
Endothelial cell migration assay.
EC migration was assessed in a similar manner. EC were serum starved for 15 h and plated in equal cell concentrations (1.5 × 105 cells/100 μl) onto 8-μm transwell inserts coated with 0.1% gelatin. SFM, Ephrin-B2/Fc (2 μg/ml), and 10% FBS were used for chemoattractants.
Nitric oxide release analysis.
Nitric oxide (NO) production by EC was analyzed as previously described (6, 18). NO production at baseline and poststimulation with VEGF-A (20 ng/ml) was examined in EphB4+/+ and EphB4+/− EC after 24 h of incubation. Collected medium was then processed by a NO-specific chemiluminescence analyzer.
Tube formation assay.
Matrigel matrix (BD Biosciences, San Jose, CA) was applied to 24-well culture plates and prepared for use. EphB4+/+ and EphB4+/− EC were plated in equal densities (2 × 105/300 μl) with or without VEGF-A (20 ng/ml) and incubated for 18 h at 37°, 5% CO2 atmosphere. Tube formation was quantified manually using light microscopy.
Western blotting.
Mouse vein and vein graft tissue were carefully harvested and snap frozen in liquid nitrogen, and protein was extracted using RIPA lysis buffer in combination with manual homogenization. Equal sample amounts of protein were fractionated in a SDS-polyacrylamide gel and then transferred to nitrocellulose. Membranes were then immunoblotted with appropriate primary and secondary antibodies, and membrane signals were detected using enhanced chemiluminescence technique.
Immunoprecipitation.
EphB4+/+ and EphB4+/− EC were serum starved for 15 h and then stimulated with 2 μg/ml of Ephrin-B2/Fc as previously described (18). After quantification of individual protein concentrations, sample concentrations were normalized and diluted to 1.5 mg/ml. Samples were incubated overnight at 4°C with anti-EphB4 antibody and then precipitated with agarose G beads. Samples were subsequently analyzed by Western blot technique. Immunoblotting was performed for phosphotyrosine.
PCR analysis.
Following serum starvation, total RNA was isolated and purified from EphB4+/+ and EphB4+/− EC (RNeasy Mini Kit; Qiagen, Valencia, CA). Total RNA was then reverse transcribed and amplified (SuperScript III First-Strand Synthesis SuperMix; Invitrogen, Grand Island, NY). Primers were used as previously described (18). Quantitative PCR was performed using duplicates of each sample, and expression values were standardized to control 18S values.
Statistical analysis.
Results are expressed as means ± SE. Comparisons between groups were performed using analysis of variance (ANOVA) or unpaired t-tests as appropriate (Prism 5, GraphPad Software). P values ≤0.05 were considered statistically significant.
RESULTS
Decreased EphB4 phosphorylation in EphB4+/− EC.
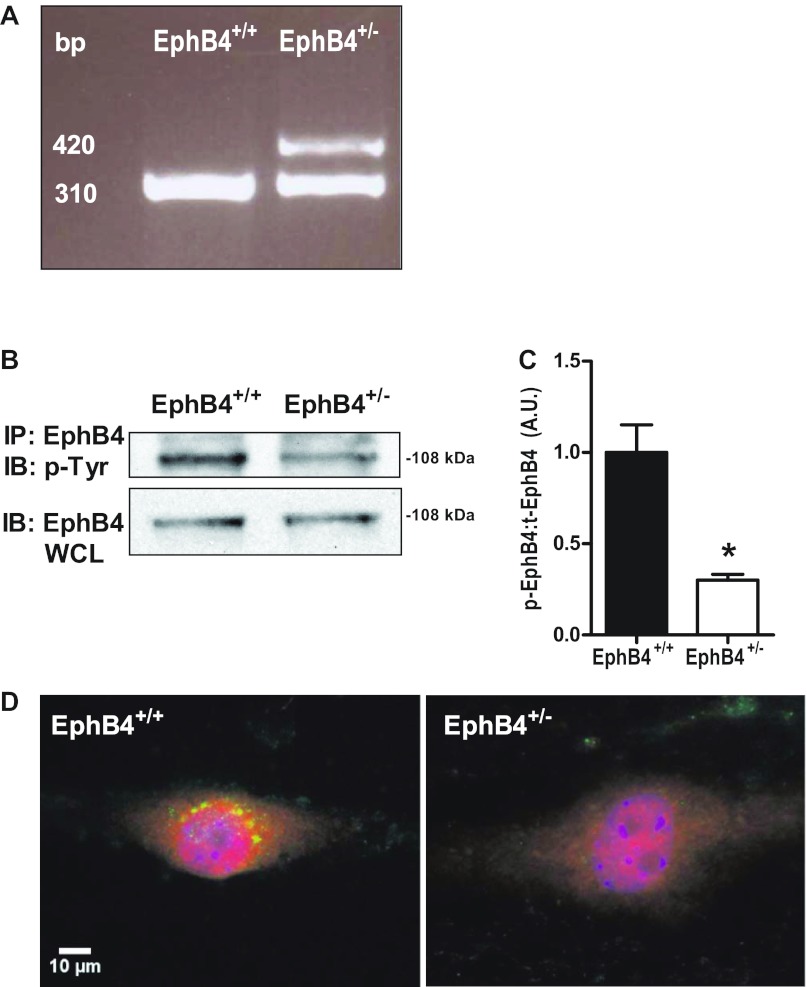
EC were isolated from both wild-type (EphB4+/+) and EphB4+/− mice; both EphB4+/+ and EphB4+/− EC expressed EphB4, consistent with a venous phenotype (Fig. 1). Germline transmission of the original EphB4 locus mutation was confirmed through extraction of total DNA from EphB4+/+ and EphB4+/− EC. Agarose gel electrophoresis demonstrated that EC isolated from EphB4+/−, but not from EphB4+/+, showed coexpression of EphB4 wild-type (310 base pairs) and EphB4 mutant (420 base pairs) loci (Fig. 1A), the same germline mutation as previously described (8). We have previously shown that EphB4+/− EC have less surface expression of EphB4, as detected with FACS, compared with EphB4+/+ EC (18). Following stimulation with Ephrin-B2/Fc, an activating ligand for EphB4 (18, 25), there was approximately threefold decreased EphB4 phosphorylation in EphB4+/− EC compared with EphB4+/+ EC (Fig. 1, B and C), consistent with reduced EphB4 function in the EphB4+/− EC. These results were confirmed with immunofluorescence staining that showed marked reduction in colocalization of EphB4 and phosphotyrosine in EphB4+/− EC following stimulation with Ephrin-B2/Fc (Fig. 1D). These results are consistent with reduced EphB4 function in EphB4+/− EC compared with EphB4+/+ EC.
Fig. 1.
Decreased EphB4 phosphorylation in EphB4+/− endothelial cells (EC). A: agarose gel electrophoresis of DNA extracted from EphB4+/+ and EphB4+/− EC shows coexpression of EphB4 wild-type (bp 310) and EphB4 mutant (bp 420) loci only in EphB4+/− EC. Representative image from n = 3 experiments is shown. B: immunoprecipitation (IP) for EphB4 with immunoblotting (IB) performed against phosphotyrosine (p-Tyr) shows diminished EphB4 phosphorylation in EphB4+/− EC (top row); whole cell lysate (WCL) shows equivalent loading (bottom row). Representative image from n = 3 experiments is shown. C: densitometry analysis of EphB4 IP. AU, arbitrary units; n = 3. *P = 0.0105. D: immunofluorescence showing diminished EphB4 phosphorylation (yellow) in EphB4+/− EC following stimulation with Ephrin-B2. Green, Eph-B4; red, phosphotyrosine; yellow, merge; blue, DAPI. Representative image from n = 3 experiments is shown. Bar, 10 μm.
FACS analysis of wild-type and EphB4+/− EC showed constitutive expression of ICAM-1 at low levels in both EphB4+/+ and EphB4+/− EC (31.80% ± 0.15% vs. 25.13% ± 2.60%, n = 3, P = 0.0628). FACS analysis for basal VCAM expression was significantly less compared with ICAM expression, and there were no differences between EphB4+/+ and EphB4+/− EC (2.13% ± 0.74% vs. 3.33% ± 0.89%, n = 3, P = 0.3592). Western blot analysis showed similar results, with no detectable differences in ICAM expression between EphB4+/+ and EphB4+/− EC (n = 4, P = 0.4113). Similarly, Western blot analysis for VCAM did not yield detectable protein levels in either EphB4+/+ and EphB4+/− EC (data not shown), consistent with the reduced expression detected with FACS. In toto, we did not observe any differences in EC phenotype between EphB4+/+ and EphB4+/− EC, consistent with our previous findings (18).
Increased VEGF-A expression in both EphB4+/− EC and vein grafts.
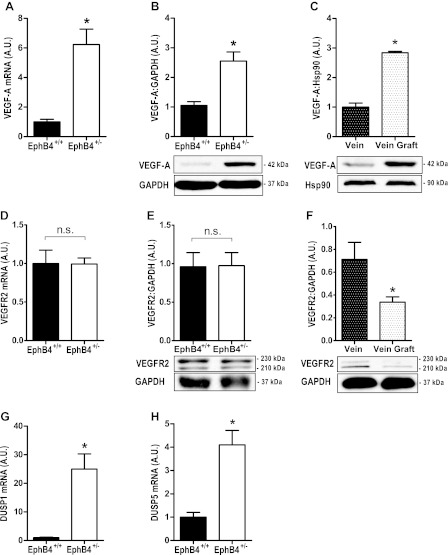
Since increased expression of VEGF-A is found during early (6–24 h) successful vein graft adaptation (12), we examined VEGF-A expression in EphB4+/+ and EphB4+/− EC. Examination of VEGF-A in unstimulated EphB4+/− EC showed increased mRNA transcripts as well as increased protein expression compared with EphB4+/+ EC (Fig. 2, A and B). Increased VEGF-A protein expression in EphB4+/− EC was similar to the increased VEGF-A protein expression in mouse vein grafts (Fig. 2C). There were no differences in VEGFR2 mRNA or protein expression between EphB4+/+ and EphB4+/− EC (Fig. 2, D and E), although there was diminished VEGFR2 protein expression in vein grafts (Fig. 2F). Analysis of EC mRNA for VEGF-A autoregulated dual-specificity MAPK phosphatases DUSP1 and DUSP5 showed increased expression levels of both DUSP1 and DUSP5 in EphB4+/− EC (Fig. 2, G and H). These results are consistent with the finding of increased VEGF-A expression in EphB4+/− EC, e.g., EphB4 is a negative regulator of VEGF-A expression in adult venous EC.
Fig. 2.
Increased VEGF-A expression in both EphB4+/− EC and vein grafts. A: bar graph of mean VEGF-A mRNA expression levels in EphB4+/+ and EphB4+/− EC (*P = 0.0006; n = 6). B: bar graph of mean densitometry of VEGF-A protein expression levels in EphB4+/+ and EphB4+/− EC (*P = 0.0106; n = 3). Representative Western blot is shown below. C: bar graph of mean densitometry of VEGF-A protein expression levels in vein and vein graft (*P = 0.0002; n = 3). Representative Western blot is shown below. D: bar graph of mean VEGFR2 mRNA expression levels in EphB4+/+ and EphB4+/− EC (*P = 0.9697; n = 6). NS, not significant. E: bar graph of mean densitometry of VEGFR2 protein expression levels in EphB4+/+ and EphB4+/− EC (*P = 0.9595, n = 6). Representative Western blot is shown below. F: bar graph of mean densitometry of VEGFR2 protein expression levels in vein and vein graft (*P = 0.0377, n = 6). Representative Western blot is shown below. G: bar graph of mean DUSP1 mRNA expression levels in EphB4+/+ and EphB4+/− EC (*P = 0.0011, n = 6). H: bar graph of mean DUSP5 mRNA expression levels in EphB4+/+ and EphB4+/− EC (*P = 0.0008, n = 6).
Reduced proliferation and migration in EphB4+/− EC.
Since VEGF-A expression was increased in EphB4+/− EC (Fig. 2), we examined ERK1/2 activity, a signaling pathway downstream of VEGF and associated with cell proliferation and migration. There were no differences in either phosphorylated or total ERK1/2 protein expression between EphB4+/+ and EphB4+/− EC (Fig. 3A). Conversely, consistent with our previous results showing increased ERK1/2 phosphorylation in rabbit vein grafts (29) and proliferation in vein grafts (12, 18), there was increased phosphorylated ERK1/2 in vein grafts compared with veins (Fig. 3B). EphB4+/− EC showed a reduced rate of proliferation compared with EphB4+/+ EC, both as assessed with direct cell counts (Fig. 3C) as well as with assessment of PCNA-positive cells (Fig. 3D). EphB4+/− EC also showed decreased migration compared with EphB4+/+ cells, both in response to Ephrin-B2/Fc as well as to FBS (Fig. 3E). These results suggest that the overall increase in ERK1/2 activity in vein grafts (Fig. 3B) is not observed in EphB4+/− EC, which show diminished proliferation and migration, and is consistent with the venous EC monolayer observed during vein graft adaptation (12, 18).
Fig. 3.
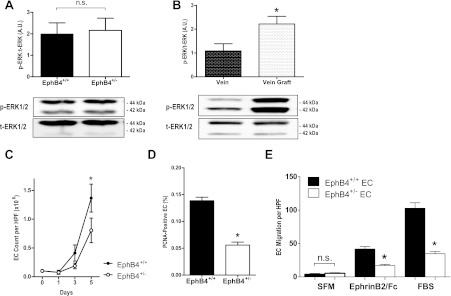
Reduced proliferation and migration in EphB4+/− EC. A: bar graph showing the ratio of mean densitometry values of phosphorylated (p) and total (t) ERK1/2 protein expression levels in EphB4+/+ and EphB4+/− EC (*P = 0.8210; n = 10). Representative Western blot is shown below. B: bar graph showing the ratio of mean densitometry values of phosphorylated and total ERK1/2 protein expression levels in vein and vein graft (*P = 0.0358; n = 3). Representative Western blot is shown below. C: line graph showing mean cell counts in EphB4+/+ (●) and EphB4+/− (○) EC over time (day 5: *P < 0.0001; n = 7). HPF, high power field. D: bar graph showing mean fraction of PCNA-positive cells in EphB4+/+ and EphB4+/− EC after 5 days in culture (*P < 0.0001; n = 6). E: bar graph showing EphB4+/+ and EphB4+/− EC migration in response to serum-free media (SFM), Ephrin-B2/Fc, or fetal bovine serum (FBS). n = 3. *P < 0.0001.
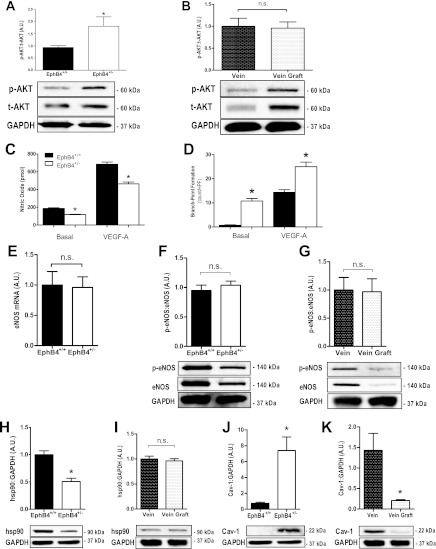
Increased angiogenesis and reduced NO production in EphB4+/− EC.
Since VEGF-A expression was increased in EphB4+/− EC (Fig. 2) but ERK1/2 activity was not (Fig. 3), we examined whether there was increased activity of the Akt pathway, which is also downstream of VEGF (7). EphB4+/− EC showed increased phosphorylated Akt, without any changes in total Akt expression, compared with EphB4+/+ EC (Fig. 4A). Vein grafts had increased expression of both phosphorylated and total Akt compared with veins (Fig. 4B). EphB4+/− EC had decreased NO secretion, both under basal as well as under VEGF-stimulated conditions, compared with EphB4+/+ EC (Fig. 4C). In contrast, EphB4+/− EC demonstrated increased tube formation with increased branch-point formation, both under basal as well under VEGF-A stimulated conditions, compared with EphB4+/+ EC (Fig. 4D).
Fig. 4.
Increased angiogenesis and reduced NO production in EphB4+/− EC. A: bar graph showing the ratio of mean densitometry values of phosphorylated and total Akt protein expression levels in EphB4+/+ and EphB4+/− EC (*P = 0.0413; n = 9). Representative Western blot is shown below. B: bar graph showing the ratio of mean densitometry values of phosphorylated and total Akt protein expression levels in vein and vein graft (n = 3). Representative Western blot is shown below. C: bar graph showing NO secretion by EphB4+/+ or EphB4+/− EC under basal (*P = 0.0012; n = 3) or VEGF-A stimulation (*P = 0.0023; n = 3). D: bar graph showing branch-point formation by EphB4+/+ or EphB4+/− EC under basal (*P < 0.0001; n = 3) or VEGF-A stimulation (*P < 0.0001; n = 3). E: bar graph showing the mean number of eNOS mRNA transcripts (P = 0.8910; n = 6). F: bar graph showing the ratio of mean densitometry values of phosphorylated and total eNOS protein expression levels in EphB4+/+ and EphB4+/− EC (*P = 0.4788; n = 3). Representative Western blot is shown below. G: bar graph showing the ratio of mean densitometry values of phosphorylated and total eNOS protein expression levels in vein and vein graft (n = 3). Representative Western blot is shown below. H: bar graph showing the mean densitometry values of hsp90 protein expression levels in EphB4+/+ and EphB4+/− EC (*P = 0.0003; n = 6). Representative Western blot is shown below. I: bar graph showing the mean densitometry values of hsp90 expression levels in vein and vein graft (P = 0.5881; n = 8). Representative Western blot is shown below. J: bar graph showing the mean densitometry values of caveolin 1 (Cav-1) protein expression levels in EphB4+/+ and EphB4+/− EC (*P = 0.0030; n = 6). Representative Western blot is shown below. K: bar graph of mean densitometry of Cav-1 protein expression levels in vein and vein graft (*P = 0.0106; n = 8). Representative Western blot is shown below.
Since both VEGF-A expression as well as phosphorylated Akt were increased in EphB4+/− EC, we examined patterns of eNOS expression. EphB4+/− EC had similar amounts of eNOS mRNA transcripts compared with EphB4+/+ EC (Fig. 4E). However, there was reduced expression of both phosphorylated and total eNOS in EphB4+/− compared with EphB4+/+ EC (Fig. 4F), similar to the reduced expression of phosphorylated and total eNOS observed in vein grafts (Fig. 4G).
Since eNOS phosphorylation and NO production were both reduced in EphB4+/− EC compared with EphB4+/+ EC (Fig. 4, F and C), we examined hsp90, a regulatory facilitator of Cav-1 displacement from eNOS. Expression of hsp90 was reduced, and expression of the eNOS inhibitor Cav-1 was elevated in EphB4+/− EC compared with EphB4+/+ EC (Fig. 4, H and J). These changes in hsp90 and Cav-1 expression were in contrast to vein grafts, in which no changes in hsp90 were observed (Fig. 4I) and Cav-1 protein levels were diminished (Fig. 4K).
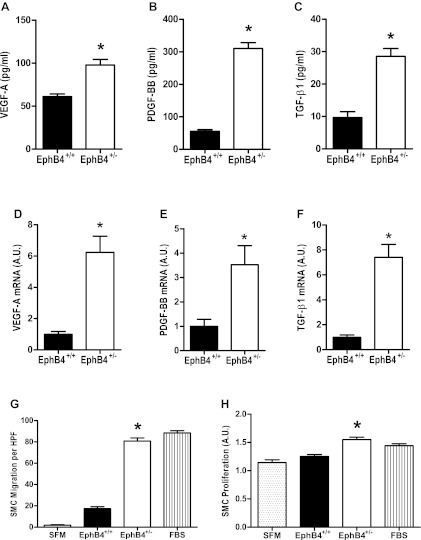
Increased SMC mitogens and chemoattractants secreted by EphB4+/− EC.
Since EphB4+/− EC have diminished proliferation and migration (Fig. 3) as well as diminished NO secretion and eNOS phosphorylation (Fig. 4), we hypothesized that EphB4+/− EC may be a functional model of the EC monolayer in vein grafts. Since vein grafts show increased neointimal volume (12, 18), increased VEGF-A expression (Fig. 2C), and ERK1/2 phosphorylation (Fig. 3B), we examined EphB4+/− EC for production of SMC mitogens and chemoattractants that could account for these observations. ELISA of EC-conditioned medium showed increased levels of VEGF-A, PDGF-BB, and TGF-β1 proteins secreted by EphB4+/− EC compared with the amounts secreted EphB4+/+ EC (Fig. 5, A–C). Similarly, mRNA transcript numbers of VEGF-A, PDGF-BB, and TGF-β1 were increased in EphB4+/− EC compared with EphB4+/+ EC (Fig. 5, D–F). SMC showed increased chemotaxis when stimulated with medium conditioned by EphB4+/− EC compared with SFM or medium conditioned by EphB4+/+ EC (Fig. 5G). Similarly, SMC stimulated with conditioned medium from EphB4+/− EC had increased proliferation compared with SMC stimulated with SFM or conditioned medium from EphB4+/+ EC (Fig. 5H). These results are consistent with EphB4+/− EC secreting increased levels of SMC chemoattractants and mitogens compared with EphB4+/+ EC.
Fig. 5.
Increased smooth muscle cell (SMC) mitogens and chemoattractants secreted by EphB4+/− EC. A–C: bar graphs showing amounts of VEGF-A (A), PDGF-BB (B), and TGF-β1 (C) protein detected in EphB4+/+ or EphB4+/− EC-conditioned medium. VEGF-A: *P = 0.0010, n = 5; PDGF-BB: *P < 0.0001, n = 6; TGF-β1: *P < 0.0001, n = 6. D–F: bar graphs showing amounts of VEGF-A (D), PDGF-BB (E), and TGF-β1 (F) mRNA detected in EphB4+/+ or EphB4+/− EC. VEGF-A: *P = 0.0006, n = 6; PDGF-BB: *P = 0.0124, n = 6; TGF-β1: *P = 0.0001, n = 6. G: bar graph showing mean number of migrating SMC (number per HPF), stimulated with either SFM, conditioned medium from either EphB4+/− or EphB4+/+ EC, or FBS. *P = 0.0358; n = 3. H: bar graph showing mean percentage of proliferating SMC (BrdU incorporation), stimulated with either SFM, conditioned medium from either EphB4+/− or EphB4+/+ EC, or FBS. *P < 0.0001; n = 7.
DISCUSSION
To examine the effects of EphB4 function in venous EC, we compared several functions of EphB4+/− EC to those of EphB4+/+ EC. Our data suggest that EphB4+/− EC have several properties that are consistently different; in particular, EphB4+/− EC have decreased rates of proliferation and migration (Fig. 3), diminished EphB4 phosphorylation (Fig. 1), increased VEGF-A synthesis (Fig. 2) and secretion (Fig. 5), increased DUSP1 and DUSP5 expression (Fig. 2), decreased eNOS expression and phosphorylation (Fig. 4) as well as decreased hsp90 expression and increased caveolin-1 expression (Fig. 4). EphB4+/− EC also demonstrate increased secretion of SMC chemoattractants and mitogens compared with EphB4+/+ EC, particularly VEGF-A, PDGF-BB, and TGF-β1 (Fig. 5). These data suggest that EphB4 has numerous effects in adult venous EC and may play an active role in cell physiology, rather than just being a passive marker of venous identity.
There are similarities between the changes in EphB4+/− EC compared with EphB4+/+ EC with those found in vein grafts compared with veins. In particular, both EphB4+/− EC as well as vein grafts show diminished EphB4 function (12, 18; Fig. 1). In addition, both EphB4+/− EC as well as vein grafts show increased VEGF-A expression (Fig. 2), increased Akt phosphorylation (Fig. 4), and diminished eNOS phosphorylation and expression (Fig. 4). We believe that these similarities reflect the altered physiological states of both EphB4+/− EC as well as vein grafts and suggest that both loss of EphB4, as well as exposure to the arterial environment in vivo, result in venous EC stress that requires an adaptive response. In particular, diminished NO and increased Akt activation suggest adaptation to stress stimuli (9, 19), or they may simply reflect diminished eNOS activation with upstream compensation. However, our examination of vein grafts at only a single time point of 1 wk after implantation suggests that additional similarities might be found at other time points. Nevertheless, our finding that reduced levels of EphB4 in EC results in several changes that recapitulate vein graft adaptation suggests that our hypothesis, that EphB4 is active in adult venous EC, is correct.
On the other hand, there are several differences between EphB4+/− EC and vein grafts, which are summarized in Table 1. Since vein grafts are composed largely of cells that are not EC, these differences suggest potential functions of vein grafts that are due to the SMC, and other non-EC cell types, rather than the EC monolayer. For example, increase ERK activation in vein grafts (Fig. 3B) suggests that vein graft proliferation is largely due to increases in SMC and other non-EC cell proliferation, consistent with the findings that vein grafts retain a single-cell EC monolayer (2, 12, 18). Examination of the vein graft at other times beyond 1 wk after implantation might also show additional similarities and differences between EphB4+/− EC and vein grafts; however, early transcriptional changes after implantation, such as for VEGF-A and EphB4, are stable between 1 and 3 wk after implantation (12).
Table 1.
Comparison of results in EphB4+/− EC to results in vein graft
| EphB4+/− EC | Vein Graft | |
|---|---|---|
| EphB4 | ↓ | ↓ |
| VEGF-A | ↑ | ↑ |
| VEGFR2 | ≈ | ↓ |
| p-ERK1/2:t-ERK1/2 | ≈ | ↑ |
| p-Akt:t-Akt | ↑ | ≈ |
| eNOS | ≈ | ≈ |
| Hsp90 | ↓ | ≈ |
| Cav-1 | ↑ | ↓ |
EC, endothelial cells; Cav-1, caveolin-1.
Diminished EphB4 function appears to result in a cellular phenotype that has some proangiogenic properties, e.g., increased VEGF-A expression, increased tube formation, and increased secretion of SMC mitogens and chemoattractants. In addition, diminished eNOS phosphorylation and NO secretion may also be consistent with this phenotype (3, 11). It is not clear whether these cellular characteristics are due to differences in activation of pathways downstream from EphB4, whether these pathways require complete suppression for phenotype emergence, or whether other factors are superimposed. The normal histology of veins in EphB4 heterozygous mice (18) suggests that reduced levels of EphB4 activity are sufficient for many of its functions, which is not surprising since complete reduction of EphB4 activity is lethal during embryonic development (27), providing rationale for redundancy of any putative functions.
Increased VEGF-A expression has been previously reported at early times during vein graft adaptation (10, 12, 28) and is consistent with our current findings in both vein grafts as well as EphB4+/− EC (Fig. 2). The significance of early increases in VEGF-A during vein graft adaptation is not well understood. VEGF-A may play a protective role in preventing intimal hyperplasia (10, 16), but it may also be a mediator of aberrant SMC proliferation and stimulus of intimal hyperplasia (28). Since VEGF-A stimulates both ERK and Akt activity (13, 14, 17, 24), our finding of differential effects on ERK (Fig. 3) and Akt (Fig. 4) activation between EphB4+/− EC and vein grafts suggests differential response to VEGF-A in the different cell types of the vein graft and is consistent with SMC having greater proliferation than EC in vein grafts. On the other hand, Akt activation in EC (Fig. 4A) but not in vein grafts (Fig. 4B) suggests the importance of the Akt pathway in EC responses as is observed with shear stress Akt activation in EC and the downstream activation of eNOS and resulting NO production (4, 26). The paracrine interactions between EC and SMC during vein graft adaptation complicate the interpretation of our vein graft data; however, our findings that EC secrete SMC mitogens and chemoattractants (Fig. 5) are consistent with a role for EphB4 in regulating this process.
Venous EC respond to shear stress and, therefore, may be the initial stimulus point for vein graft adaptation to the arterial circulation (15). We believe that EphB4 expression on venous EC is a critical modulator of vein graft adaptation (12); diminished EphB4 function results in increased wall thickening in EphB4-heterozygous vein grafts (18), consistent with our finding of a proangiogenic and mitogenic phenotype in EphB4+/− EC. In toto, these data suggest that EphB4 is functional in adult venous EC.
GRANTS
This work was supported by the National Institutes of Health (R01-HL095498-01 to A. Dardik); the American Vascular Association William J. von Liebig Award (to A. Dardik); the Hartford Hospital Ludwig J. Pyrtek Fund (to C. C. Jadlowiec); the Japan Society for the Promotion of Science (JSPS) KAKENHI Grant 24390299 (to A. Muto); as well as with the resources and the use of facilities at the VA Connecticut Healthcare System, West Haven, CT.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
C.C.J., A.F., and A.D. conception and design of the research; C.C.J., A.F., C.Y., A.J.F., S.T.K., M.J.C., Y.K., and A.M. performed the experiments; C.C.J., A.M., and A.D. analyzed the data; C.C.J., C.Y., A.J.F., Y.K., A.M., and A.D. interpreted the results of the experiments; C.C.J. and A.D. prepared the figures; C.C.J. and A.D. drafted the manuscript; C.C.J., A.F., C.Y., A.J.F., S.T.K., M.J.C., Y.K., A.M., and A.D. edited and revised the manuscript; C.C.J., A.F., C.Y., A.J.F., S.T.K., M.J.C., Y.K., A.M., and A.D. approved the final version of the manuscript.
REFERENCES
- 1.Adams RH, Wilkinson GA, Weiss C, Diella F, Gale NW, Deutsch U, Risau W, Klein R. Roles of ephrinB ligands and EphB receptors in cardiovascular development: demarcation of arterial/venous domains, vascular morphogenesis, and sprouting angiogenesis. Genes Dev 13: 295–306, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen KE, Varty K, Jones L, Sayers RD, Bell PR, London NJ. Human venous endothelium can promote intimal hyperplasia in a paracrine manner. J Vasc Surg 19: 577–584, 1994 [DOI] [PubMed] [Google Scholar]
- 3.Cayatte AJ, Palacino JJ, Horten K, Cohen RA. Chronic inhibition of nitric oxide production accelerates neointima formation and impairs endothelial function in hypercholesterolemic rabbits. Arterioscler Thromb 14: 753–759, 1994 [DOI] [PubMed] [Google Scholar]
- 4.Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature 399: 601–605, 1999 [DOI] [PubMed] [Google Scholar]
- 5.Fernández-Hernando C, József L, Jenkins D, Di Lorenzo A, Sessa WC. Absence of Akt1 reduces vascular smooth muscle cell migration and survival and induces features of plaque vulnerability and cardiac dysfunction during atherosclerosis. Arterioscler Thromb Vasc Biol 29: 2033–2040, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fernández-Hernando C, Fukata M, Bernatchez PN, Fukata Y, Lin MI, Bredt DS, Sessa WC. Identification of Golgi-localized acyl transferases that palmitoylate and regulate endothelial nitric oxide synthase. J Cell Biol 174: 369–377, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gerber HP, McMurtrey A, Kowalski J, Yan M, Keyt BA, Dixit V, Ferrara N. Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3′-kinase/Akt signal transduction pathway. Requirement for Flk-1/KDR activation. J Biol Chem 273: 30336–30343, 1998 [DOI] [PubMed] [Google Scholar]
- 8.Gerety SS, Wang HU, Chen ZF, Anderson DJ. Symmetrical mutant phenotypes of the receptor EphB4 and its specific transmembrane ligand ephrin-B2 in cardiovascular development. Mol Cell 4: 403–414, 1999 [DOI] [PubMed] [Google Scholar]
- 9.Hata JA, Petrofski JA, Schroder JN, Williams ML, Timberlake SH, Pippen A, Corwin MT, Solan AK, Jakoi A, Gehrig TR, Kontos CD, Milano CA. Modulation of phosphatidylinositol 3-kinase signaling reduces intimal hyperplasia in aortocoronary saphenous vein grafts. J Thorac Cardiovasc Surg 129: 1405–1413, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Hamdan AD, Aiello LP, Misare BD, Contreras MA, King GL, LoGerfo FW, Quist WC. Vascular endothelial growth factor expression in canine peripheral vein bypass grafts. J Vasc Surg 26: 79–86, 1997 [DOI] [PubMed] [Google Scholar]
- 11.Janssens S, Flaherty D, Nong Z, Varenne O, van Pelt N, Haustermans C, Zoldhelyi P, Gerard R, Collen D. Human endothelial nitric oxide synthase gene transfer inhibits vascular smooth muscle cell proliferation and neointima formation after balloon injury in rats. Circulation 97: 1274–1281, 1998 [DOI] [PubMed] [Google Scholar]
- 12.Kudo FA, Muto A, Maloney SP, Pimiento JM, Bergaya S, Fitzgerald TN, Westvik TS, Frattini JC, Breuer CK, Cha CH, Nishibe T, Tellides G, Sessa WC, Dardik A. Venous identity is lost but arterial identity is not gained during vein graft adaptation. Arterioscler Thromb Vasc Biol 27: 1562–1571, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Lal BK, Varma S, Pappas PJ, Hobson RW, 2nd, Durán WN. VEGF increases permeability of the endothelial cell monolayer by activation of PKB/akt, endothelial nitric-oxide synthase, and MAP kinase pathways. Microvasc Res 62: 252–262, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Lee JH, Johnson PR, Roth M, Hunt NH, Black JL. ERK activation and mitogenesis in human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 280: L1019–L1029, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Li YS, Haga JH, Chien S. Molecular basis of the effects of shear stress on vascular endothelial cells. J Biomech 38: 1949–1971, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Luo Z, Asahara T, Tsurumi Y, Isner JM, Symes JF. Reduction of vein graft intimal hyperplasia and preservation of endothelium-dependent relaxation by topical vascular endothelial growth factor. J Vasc Surg 27: 167–173, 1998 [DOI] [PubMed] [Google Scholar]
- 17.Morales-Ruiz M, Fulton D, Sowa G, Languino LR, Fujio Y, Walsh K, Sessa WC. Vascular endothelial growth factor-stimulated actin reorganization and migration of endothelial cells is regulated via the serine/threonine kinase Akt. Circ Res 86: 892–896, 2000 [DOI] [PubMed] [Google Scholar]
- 18.Muto A, Yi T, Harrison KD, Dávalos A, Fancher TT, Ziegler KR, Feigel A, Kondo Y, Nishibe T, Sessa WC, Dardik A. Eph-B4 prevents venous adaptive remodeling in the adult arterial environment. J Exp Med 208: 561–575, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Niu XF, Smith CW, Kubes P. Intracellular oxidative stress induced by nitric oxide synthesis inhibition increases endothelial cell adhesion to neutrophils. Circ Res 74: 1133–1140, 1994 [DOI] [PubMed] [Google Scholar]
- 20.Owens CD, Wake N, Jacot JG, Gerhard-Herman M, Gaccione P, Belkin M, Creager MA, Conte Owens CD MS, Wake N, Jacot JG, Gerhard-Herman M, Gaccione P, Belkin M, Creager MA, Conte MS. Early biomechanical changes in lower extremity vein grafts–distinct temporal phases of remodeling and wall stiffness. J Vasc Surg 44: 740–746, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Owens CD, Rybicki FJ, Wake N, Schanzer A, Mitsouras D, Gerhard-Herman MD, Conte MS. Early remodeling of lower extremity vein grafts: inflammation influences biomechanical adaptation. J Vasc Surg 47: 1235–1242, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Owens CD. Adaptive changes in autogenous vein grafts for arterial reconstruction: clinical implications. J Vasc Surg 51: 736–746, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shah DM, Darling RC, 3rd, Chang BB, Fitzgerald KM, Paty PS, Leather RP. Long-term results of in situ saphenous vein bypass. Analysis of 2058 cases. Ann Surg 222: 438–446, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Srinivasan R, Zabuawala T, Huang H, Zhang J, Gulati P, Fernandez S, Karlo JC, Landreth GE, Leone G, Ostrowski MC. Erk1 and Erk2 regulate endothelial cell proliferation and migration during mouse embryonic angiogenesis. PLos One 4: e8283, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Steinle JJ, Meininger CJ, Forough R, Wu G, Wu MH, Granger HJ. Eph B4 receptor signaling mediates endothelial cell migration and proliferation via the phosphatidylinositol 3-kinase pathway. J Biol Chem 277: 43830–43835, 2002 [DOI] [PubMed] [Google Scholar]
- 26.van der Zee R, Murohara T, Luo Z, Zollmann F, Passeri J, Lekutat C, Isner JM. Vascular endothelial growth factor/vascular permeability actor augments nitric oxide release from quiescent rabbit and human vascular endothelium. Circulation 95: 1030–1037, 1997 [DOI] [PubMed] [Google Scholar]
- 27.Wang HU, Chen ZF, Anderson DJ. Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4. Cell 93: 741–753, 1998 [DOI] [PubMed] [Google Scholar]
- 28.Westerband A, Gentile AT, Hunter GC, Gooden MA, Aguirre ML, Berman SS, Mills JL. Intimal growth and neovascularization in human stenotic vein grafts. J Am Coll Surg 191: 264–271, 2000 [DOI] [PubMed] [Google Scholar]
- 29.Yamashita A, Hanna AK, Hirata S, Dardik A, Sumpio BE. Antisense basic fibroblast growth factor alters the time course of mitogen-activated protein kinase in arterialized vein graft remodeling. J Vasc Surg 37: 866–873, 2003 [DOI] [PubMed] [Google Scholar]