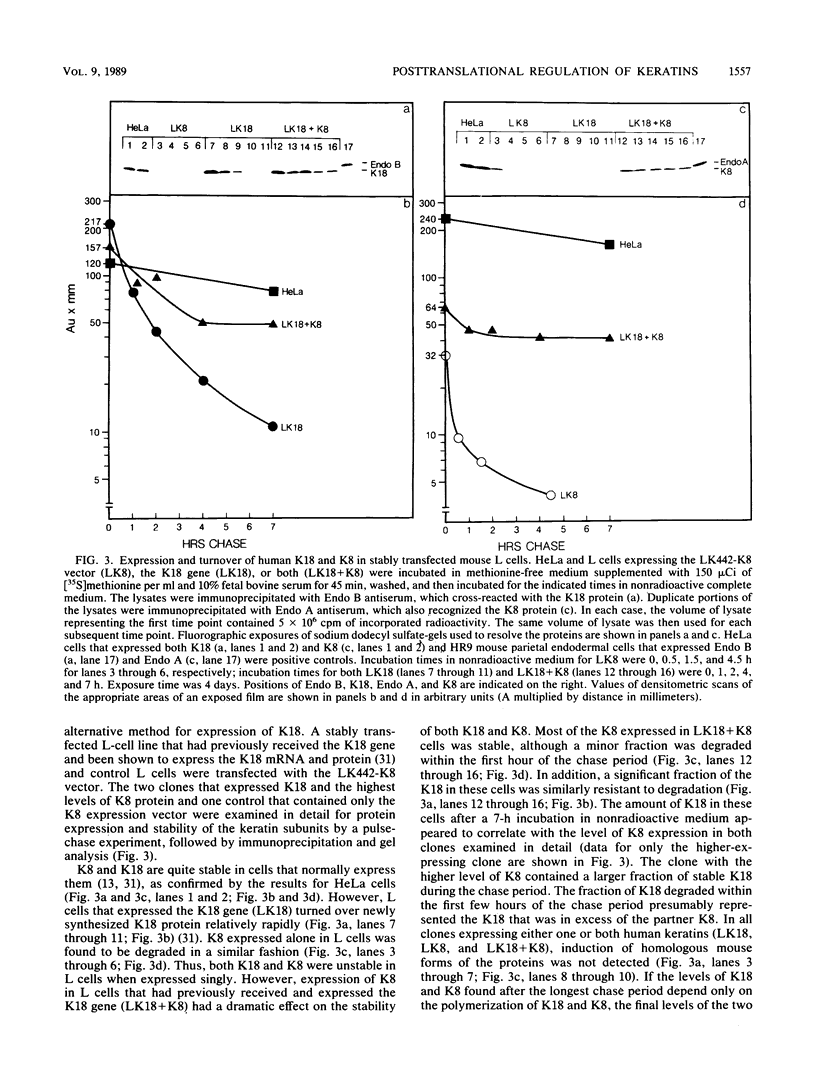
Abstract
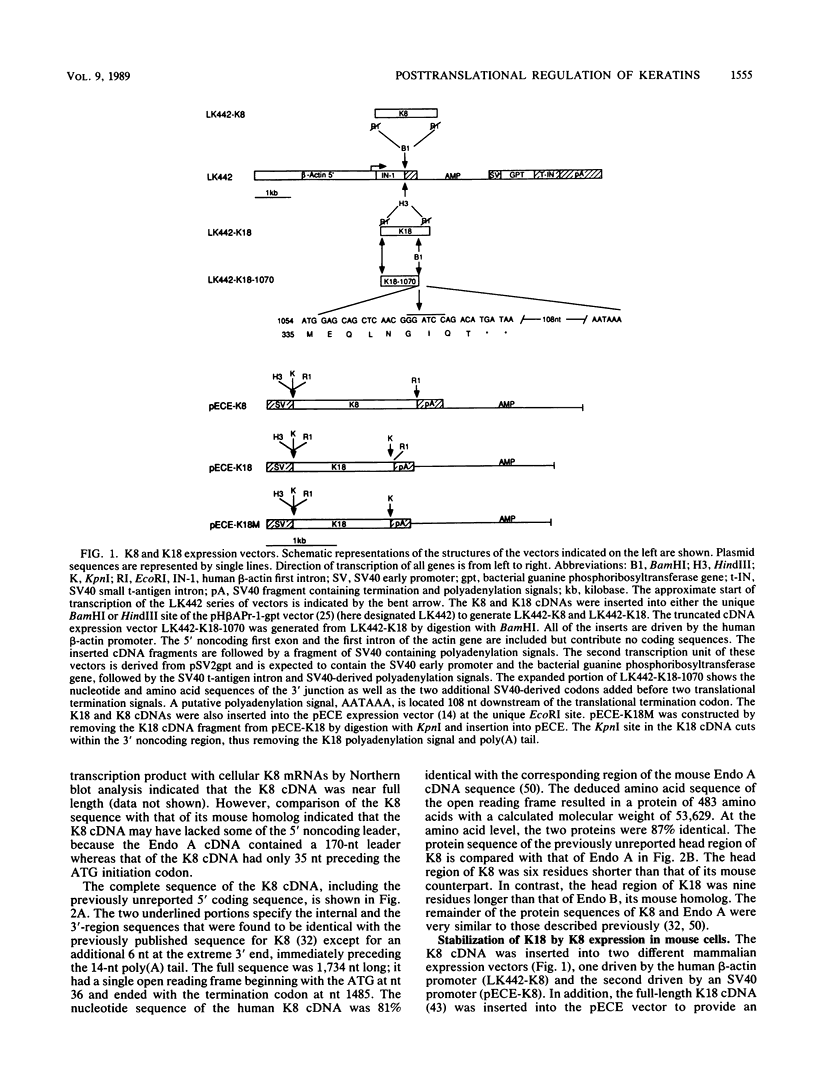
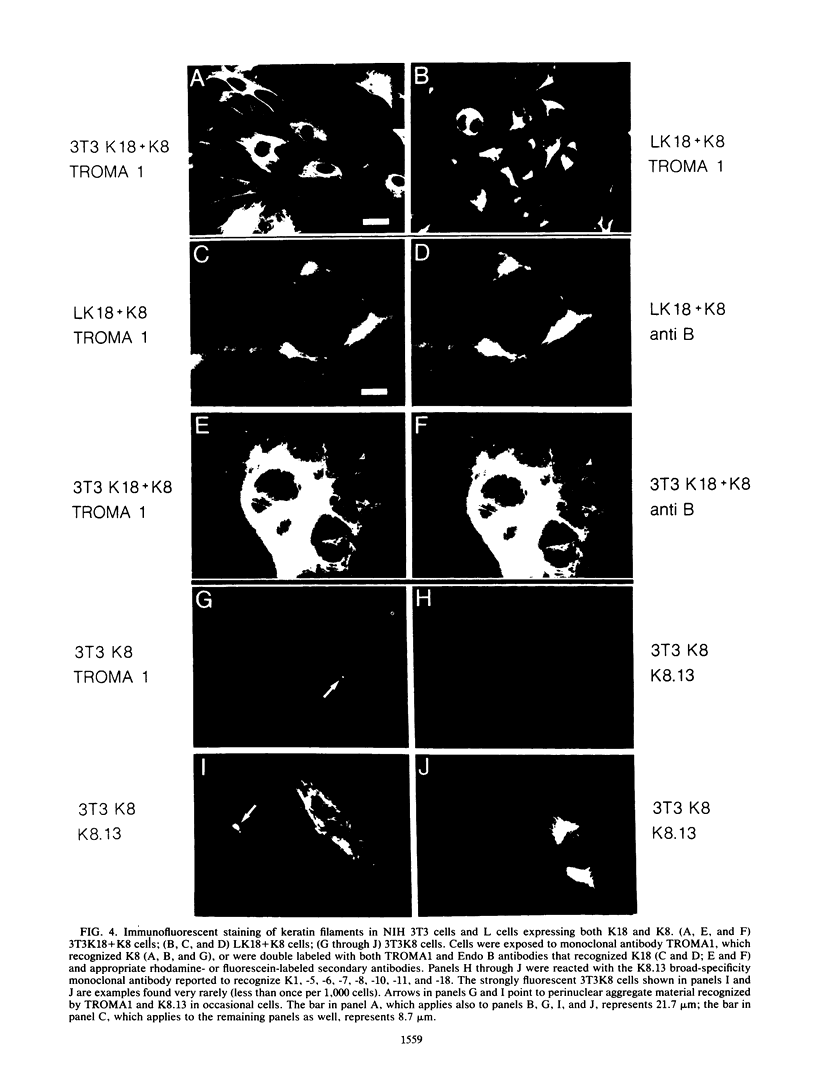
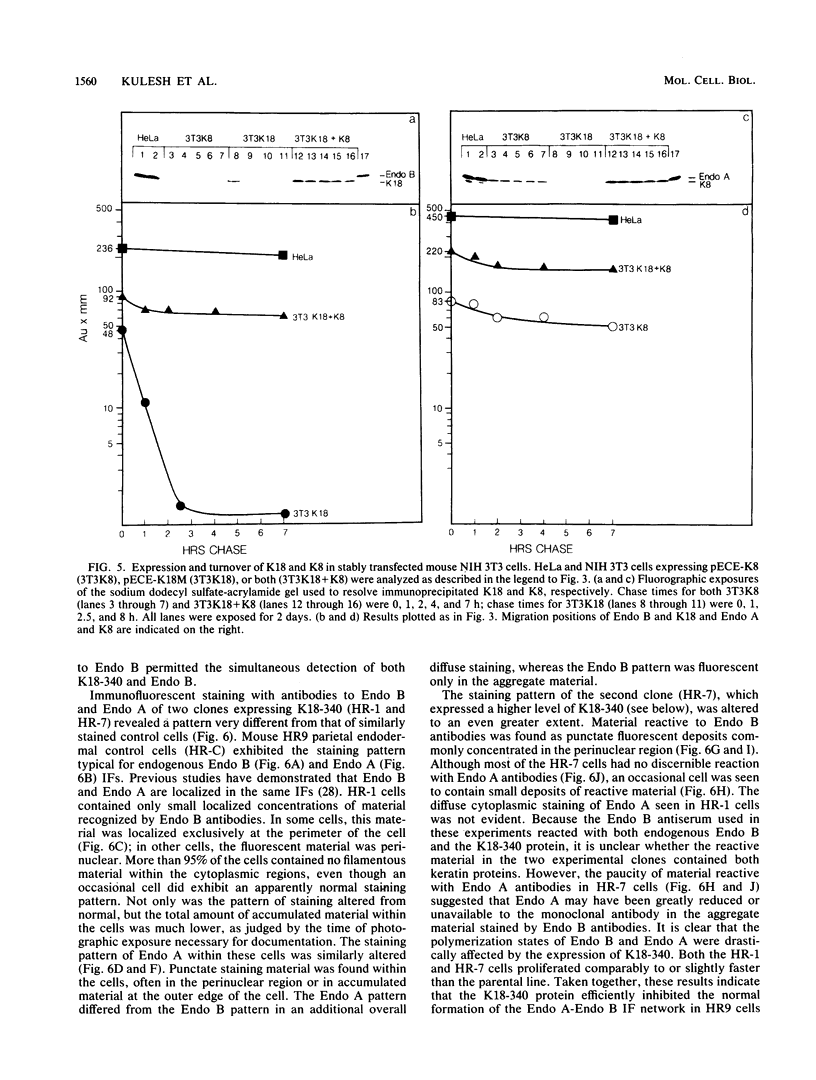
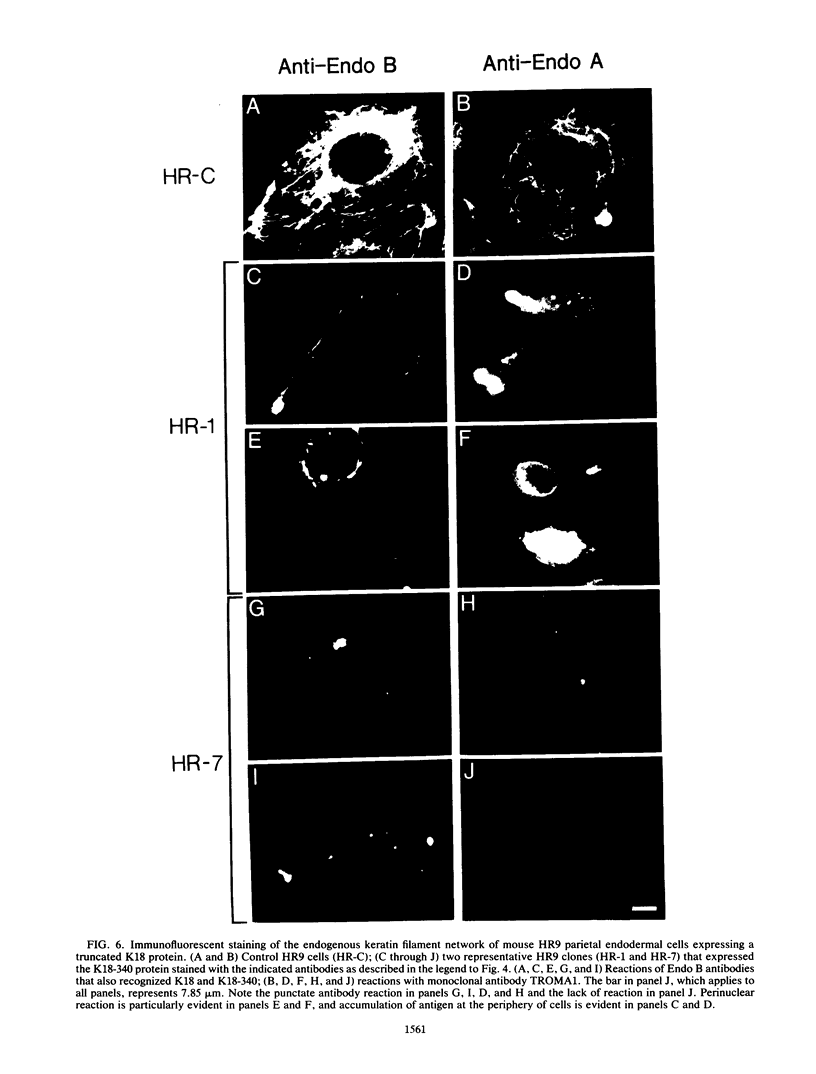
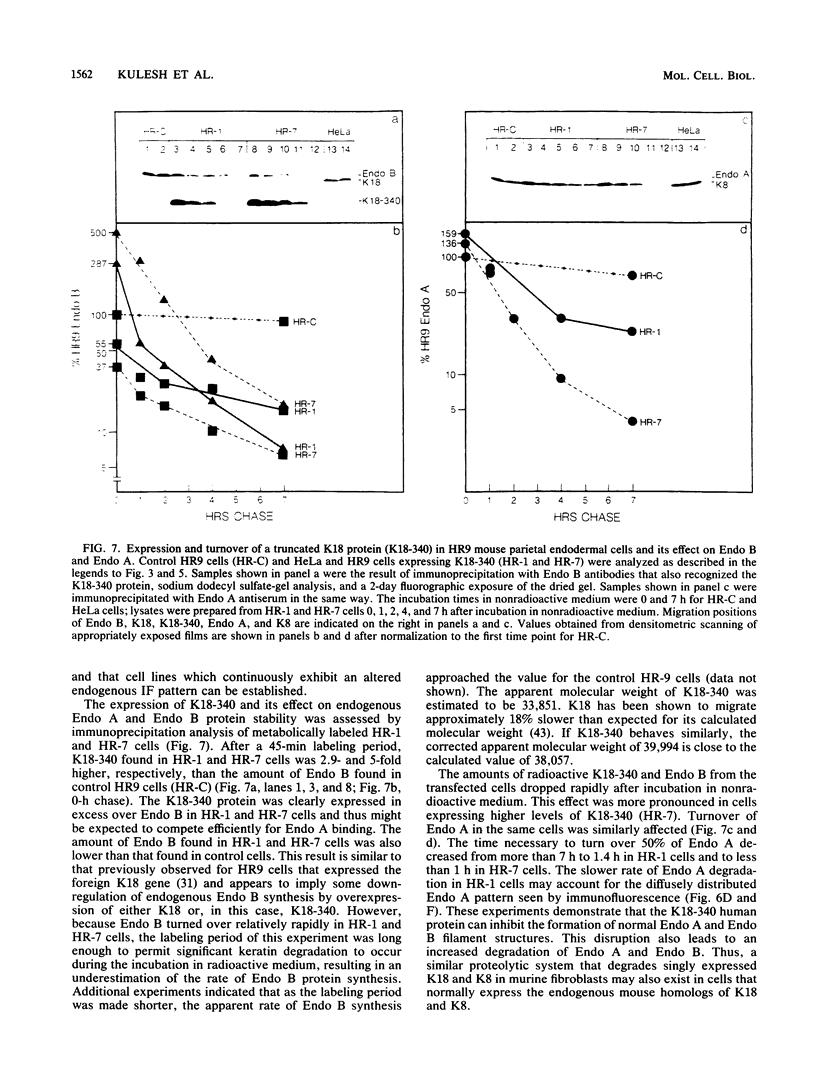
Human keratin 18 (K18) and keratin 8 (K8) and their mouse homologs, Endo B and Endo A, respectively, are expressed in adult mice primarily in a variety of simple epithelial cell types in which they are normally found in equal amounts within the intermediate filament cytoskeleton. Expression of K18 alone in mouse L cells or NIH 3T3 fibroblasts from either the gene or a cDNA expression vector results in K18 protein which is degraded relatively rapidly without the formation of filaments. A K8 cDNA containing all coding sequences was isolated and expressed in mouse fibroblasts either singly or in combination with K18. Immunoprecipitation of stably transfected L cells revealed that when K8 was expressed alone, it was degraded in a fashion similar to that seen previously for K18. However, expression of K8 in fibroblasts that also expressed K18 resulted in stabilization of both K18 and K8. Immunofluorescent staining revealed typical keratin filament organization in such cells. Thus, expression of a type I and a type II keratin was found to be both necessary and sufficient for formation of keratin filaments within fibroblasts. To determine whether a similar proteolytic system responsible for the degradation of K18 in fibroblasts also exists in simple epithelial cells which normally express a type I and a type II keratin, a mutant, truncated K18 protein missing the carboxy-terminal tail domain and a conserved region of the central, alpha-helical rod domain was expressed in mouse parietal endodermal cells. This resulted in destabilization of endogenous Endo A and Endo B and inhibition of the formation of typical keratin filament structures. Therefore, cells that normally express keratins contain a proteolytic system similar to that found in experimentally manipulated fibroblasts which degrades keratin proteins not found in their normal polymerized state.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albers K., Fuchs E. The expression of mutant epidermal keratin cDNAs transfected in simple epithelial and squamous cell carcinoma lines. J Cell Biol. 1987 Aug;105(2):791–806. doi: 10.1083/jcb.105.2.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggin M. D., Gibson T. J., Hong G. F. Buffer gradient gels and 35S label as an aid to rapid DNA sequence determination. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3963–3965. doi: 10.1073/pnas.80.13.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch F. X., Leube R. E., Achtstätter T., Moll R., Franke W. W. Expression of simple epithelial type cytokeratins in stratified epithelia as detected by immunolocalization and hybridization in situ. J Cell Biol. 1988 May;106(5):1635–1648. doi: 10.1083/jcb.106.5.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brûlet P., Babinet C., Kemler R., Jacob F. Monoclonal antibodies against trophectoderm-specific markers during mouse blastocyst formation. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4113–4117. doi: 10.1073/pnas.77.7.4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brûlet P., Duprey P., Vasseur M., Kaghad M., Morello D., Blanchet P., Babinet C., Condamine H., Jacob F. Molecular analysis of the first differentiations in the mouse embryo. Cold Spring Harb Symp Quant Biol. 1985;50:51–57. doi: 10.1101/sqb.1985.050.01.009. [DOI] [PubMed] [Google Scholar]
- Cabral F., Gottesman M. M., Zimmerman S. B., Steinert P. M. Intermediate filaments from Chinese hamster ovary cells contain a single protein. Comparison with more complex systems from baby hamster kidney and mouse epidermal cells. J Biol Chem. 1981 Feb 10;256(3):1428–1431. [PubMed] [Google Scholar]
- Chisholm J. C., Houliston E. Cytokeratin filament assembly in the preimplantation mouse embryo. Development. 1987 Nov;101(3):565–582. doi: 10.1242/dev.101.3.565. [DOI] [PubMed] [Google Scholar]
- Chung A. E., Estes L. E., Shinozuka H., Braginski J., Lorz C., Chung C. A. Morphological and biochemical observations on cells derived from the in vitro differentiation of the embryonal carcinoma cell line PCC4-F. Cancer Res. 1977 Jul;37(7 Pt 1):2072–2081. [PubMed] [Google Scholar]
- Darmon M., Nicolas J. F., Lamblin D. 5-Azacytidine is able to induce the conversion of teratocarcinoma-derived mesenchymal cells into epithelia cells. EMBO J. 1984 May;3(5):961–967. doi: 10.1002/j.1460-2075.1984.tb01914.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denk H., Franke W. W., Eckerstorfer R., Schmid E., Kerjaschki D. Formation and involution of Mallory bodies ("alcoholic hyalin") in murine and human liver revealed by immunofluorescence microscopy with antibodies to prekeratin. Proc Natl Acad Sci U S A. 1979 Aug;76(8):4112–4116. doi: 10.1073/pnas.76.8.4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denk H., Lackinger E., Zatloukal K., Franke W. W. Turnover of cytokeratin polypeptides in mouse hepatocytes. Exp Cell Res. 1987 Nov;173(1):137–143. doi: 10.1016/0014-4827(87)90339-9. [DOI] [PubMed] [Google Scholar]
- Domenjoud L., Jorcano J. L., Breuer B., Alonso A. Synthesis and fate of keratins 8 and 18 in nonepithelial cells transfected with cDNA. Exp Cell Res. 1988 Dec;179(2):352–361. doi: 10.1016/0014-4827(88)90274-1. [DOI] [PubMed] [Google Scholar]
- Ellis L., Clauser E., Morgan D. O., Edery M., Roth R. A., Rutter W. J. Replacement of insulin receptor tyrosine residues 1162 and 1163 compromises insulin-stimulated kinase activity and uptake of 2-deoxyglucose. Cell. 1986 Jun 6;45(5):721–732. doi: 10.1016/0092-8674(86)90786-5. [DOI] [PubMed] [Google Scholar]
- Emerson J. A. Disruption of the cytokeratin filament network in the preimplantation mouse embryo. Development. 1988 Oct;104(2):219–234. doi: 10.1242/dev.104.2.219. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Denk H., Kalt R., Schmid E. Biochemical and immunological identification of cytokeratin proteins present in hepatocytes of mammalian liver tissue. Exp Cell Res. 1981 Feb;131(2):299–318. doi: 10.1016/0014-4827(81)90234-2. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Denk H., Schmid E., Osborn M., Weber K. Ultrastructural, biochemical, and immunologic characterization of Mallory bodies in livers of griseofulvin-treated mice. Fimbriated rods of filaments containing prekeratin-like polypeptides. Lab Invest. 1979 Feb;40(2):207–220. [PubMed] [Google Scholar]
- Franke W. W., Schiller D. L., Moll R., Winter S., Schmid E., Engelbrecht I., Denk H., Krepler R., Platzer B. Diversity of cytokeratins. Differentiation specific expression of cytokeratin polypeptides in epithelial cells and tissues. J Mol Biol. 1981 Dec 25;153(4):933–959. doi: 10.1016/0022-2836(81)90460-5. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Schmid E., Grund C., Geiger B. Intermediate filament proteins in nonfilamentous structures: transient disintegration and inclusion of subunit proteins in granular aggregates. Cell. 1982 Aug;30(1):103–113. doi: 10.1016/0092-8674(82)90016-2. [DOI] [PubMed] [Google Scholar]
- Geisler N., Weber K. Comparison of the proteins of two immunologically distinct intermediate-sized filaments by amino acid sequence analysis: desmin and vimentin. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4120–4123. doi: 10.1073/pnas.78.7.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler N., Weber K. Self-assembly in Vitro of the 68,000 molecular weight component of the mammalian neurofilament triplet proteins into intermediate-sized filaments. J Mol Biol. 1981 Sep 25;151(3):565–571. doi: 10.1016/0022-2836(81)90011-5. [DOI] [PubMed] [Google Scholar]
- Gigi O., Geiger B., Eshhar Z., Moll R., Schmid E., Winter S., Schiller D. L., Franke W. W. Detection of a cytokeratin determinant common to diverse epithelial cells by a broadly cross-reacting monoclonal antibody. EMBO J. 1982;1(11):1429–1437. doi: 10.1002/j.1460-2075.1982.tb01334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giudice G. J., Fuchs E. The transfection of epidermal keratin genes into fibroblasts and simple epithelial cells: evidence for inducing a type I keratin by a type II gene. Cell. 1987 Feb 13;48(3):453–463. doi: 10.1016/0092-8674(87)90196-6. [DOI] [PubMed] [Google Scholar]
- Gronostajski R. M., Goldberg A. L., Pardee A. B. Energy requirement for degradation of tumor-associated protein p53. Mol Cell Biol. 1984 Mar;4(3):442–448. doi: 10.1128/mcb.4.3.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunning P., Leavitt J., Muscat G., Ng S. Y., Kedes L. A human beta-actin expression vector system directs high-level accumulation of antisense transcripts. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4831–4835. doi: 10.1073/pnas.84.14.4831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatzfeld M., Franke W. W. Pair formation and promiscuity of cytokeratins: formation in vitro of heterotypic complexes and intermediate-sized filaments by homologous and heterologous recombinations of purified polypeptides. J Cell Biol. 1985 Nov;101(5 Pt 1):1826–1841. doi: 10.1083/jcb.101.5.1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herskowitz I. Functional inactivation of genes by dominant negative mutations. Nature. 1987 Sep 17;329(6136):219–222. doi: 10.1038/329219a0. [DOI] [PubMed] [Google Scholar]
- Howe W. E., Klier F. G., Oshima R. G. Murine endodermal cytokeratins Endo A and Endo B are localized in the same intermediate filament. J Histochem Cytochem. 1986 Jun;34(6):785–793. doi: 10.1177/34.6.2422254. [DOI] [PubMed] [Google Scholar]
- Ichinose Y., Morita T., Zhang F. Y., Srimahasongcram S., Tondella M. L., Matsumoto M., Nozaki M., Matsushiro A. Nucleotide sequence and structure of the mouse cytokeratin endoB gene. Gene. 1988 Oct 15;70(1):85–95. doi: 10.1016/0378-1119(88)90107-2. [DOI] [PubMed] [Google Scholar]
- Klymkowsky M. W., Miller R. H., Lane E. B. Morphology, behavior, and interaction of cultured epithelial cells after the antibody-induced disruption of keratin filament organization. J Cell Biol. 1983 Feb;96(2):494–509. doi: 10.1083/jcb.96.2.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulesh D. A., Oshima R. G. Cloning of the human keratin 18 gene and its expression in nonepithelial mouse cells. Mol Cell Biol. 1988 Apr;8(4):1540–1550. doi: 10.1128/mcb.8.4.1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leube R. E., Bosch F. X., Romano V., Zimbelmann R., Höfler H., Franke W. W. Cytokeratin expression in simple epithelia. III. Detection of mRNAs encoding human cytokeratins nos. 8 and 18 in normal and tumor cells by hybridization with cDNA sequences in vitro and in situ. Differentiation. 1986;33(1):69–85. doi: 10.1111/j.1432-0436.1986.tb00412.x. [DOI] [PubMed] [Google Scholar]
- Liem R. K., Hutchison S. B. Purification of individual components of the neurofilament triplet: filament assembly from the 70 000-dalton subunit. Biochemistry. 1982 Jun 22;21(13):3221–3226. doi: 10.1021/bi00256a029. [DOI] [PubMed] [Google Scholar]
- Magin T. M., Hatzfeld M., Franke W. W. Analysis of cytokeratin domains by cloning and expression of intact and deleted polypeptides in Escherichia coli. EMBO J. 1987 Sep;6(9):2607–2615. doi: 10.1002/j.1460-2075.1987.tb02551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millán J. L. Molecular cloning and sequence analysis of human placental alkaline phosphatase. J Biol Chem. 1986 Mar 5;261(7):3112–3115. [PubMed] [Google Scholar]
- Mizusawa S., Nishimura S., Seela F. Improvement of the dideoxy chain termination method of DNA sequencing by use of deoxy-7-deazaguanosine triphosphate in place of dGTP. Nucleic Acids Res. 1986 Feb 11;14(3):1319–1324. doi: 10.1093/nar/14.3.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll R., Franke W. W., Schiller D. L., Geiger B., Krepler R. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell. 1982 Nov;31(1):11–24. doi: 10.1016/0092-8674(82)90400-7. [DOI] [PubMed] [Google Scholar]
- Moon H. M., Wisniewski T., Merz P., De Martini J., Wisniewski H. M. Partial purification of neurofilament subunits from bovine brains and studies on neurofilament assembly. J Cell Biol. 1981 Jun;89(3):560–567. doi: 10.1083/jcb.89.3.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan R. C., Berg P. Selection for animal cells that express the Escherichia coli gene coding for xanthine-guanine phosphoribosyltransferase. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2072–2076. doi: 10.1073/pnas.78.4.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshima R. G. Developmental expression of murine extra-embryonic endodermal cytoskeletal proteins. J Biol Chem. 1982 Apr 10;257(7):3414–3421. [PubMed] [Google Scholar]
- Oshima R. G., Howe W. E., Klier F. G., Adamson E. D., Shevinsky L. H. Intermediate filament protein synthesis in preimplantation murine embryos. Dev Biol. 1983 Oct;99(2):447–455. doi: 10.1016/0012-1606(83)90294-4. [DOI] [PubMed] [Google Scholar]
- Oshima R. G. Identification and immunoprecipitation of cytoskeletal proteins from murine extra-embryonic endodermal cells. J Biol Chem. 1981 Aug 10;256(15):8124–8133. [PubMed] [Google Scholar]
- Oshima R. G., Millán J. L., Ceceña G. Comparison of mouse and human keratin 18: a component of intermediate filaments expressed prior to implantation. Differentiation. 1986;33(1):61–68. doi: 10.1111/j.1432-0436.1986.tb00411.x. [DOI] [PubMed] [Google Scholar]
- Oshima R. G., Trevor K., Shevinsky L. H., Ryder O. A., Ceceña G. Identification of the gene coding for the Endo B murine cytokeratin and its methylated, stable inactive state in mouse nonepithelial cells. Genes Dev. 1988 May;2(5):505–516. doi: 10.1101/gad.2.5.505. [DOI] [PubMed] [Google Scholar]
- Quinlan R. A., Cohlberg J. A., Schiller D. L., Hatzfeld M., Franke W. W. Heterotypic tetramer (A2D2) complexes of non-epidermal keratins isolated from cytoskeletons of rat hepatocytes and hepatoma cells. J Mol Biol. 1984 Sep 15;178(2):365–388. doi: 10.1016/0022-2836(84)90149-9. [DOI] [PubMed] [Google Scholar]
- Quinlan R. A., Franke W. W. Heteropolymer filaments of vimentin and desmin in vascular smooth muscle tissue and cultured baby hamster kidney cells demonstrated by chemical crosslinking. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3452–3456. doi: 10.1073/pnas.79.11.3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan R. A., Franke W. W. Molecular interactions in intermediate-sized filaments revealed by chemical cross-linking. Heteropolymers of vimentin and glial filament protein in cultured human glioma cells. Eur J Biochem. 1983 May 16;132(3):477–484. doi: 10.1111/j.1432-1033.1983.tb07386.x. [DOI] [PubMed] [Google Scholar]
- Rogers S., Wells R., Rechsteiner M. Amino acid sequences common to rapidly degraded proteins: the PEST hypothesis. Science. 1986 Oct 17;234(4774):364–368. doi: 10.1126/science.2876518. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer P. A., Trevor K., Oshima R. G. Molecular cloning and characterization of the Endo B cytokeratin expressed in preimplantation mouse embryos. J Biol Chem. 1986 Jan 15;261(2):538–547. [PubMed] [Google Scholar]
- Steinert P. M., Idler W. W., Cabral F., Gottesman M. M., Goldman R. D. In vitro assembly of homopolymer and copolymer filaments from intermediate filament subunits of muscle and fibroblastic cells. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3692–3696. doi: 10.1073/pnas.78.6.3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinert P. M., Idler W. W., Zimmerman S. B. Self-assembly of bovine epidermal keratin filaments in vitro. J Mol Biol. 1976 Dec 15;108(3):547–567. doi: 10.1016/s0022-2836(76)80136-2. [DOI] [PubMed] [Google Scholar]
- Steinert P. M., Parry D. A. Intermediate filaments: conformity and diversity of expression and structure. Annu Rev Cell Biol. 1985;1:41–65. doi: 10.1146/annurev.cb.01.110185.000353. [DOI] [PubMed] [Google Scholar]
- Steinert P. M., Roop D. R. Molecular and cellular biology of intermediate filaments. Annu Rev Biochem. 1988;57:593–625. doi: 10.1146/annurev.bi.57.070188.003113. [DOI] [PubMed] [Google Scholar]
- Sémat A., Vasseur M., Maillet L., Brûlet P., Darmon Y. M. Sequence analysis of murine cytokeratin endo A (no. 8) cDNA. Evidence for mRNA species initiated upstream of the normal 5' end in PCC4 cells. Differentiation. 1988;37(1):40–46. doi: 10.1111/j.1432-0436.1988.tb00794.x. [DOI] [PubMed] [Google Scholar]
- Vasseur M., Duprey P., Brûlet P., Jacob F. One gene and one pseudogene for the cytokeratin endo A. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1155–1159. doi: 10.1073/pnas.82.4.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]