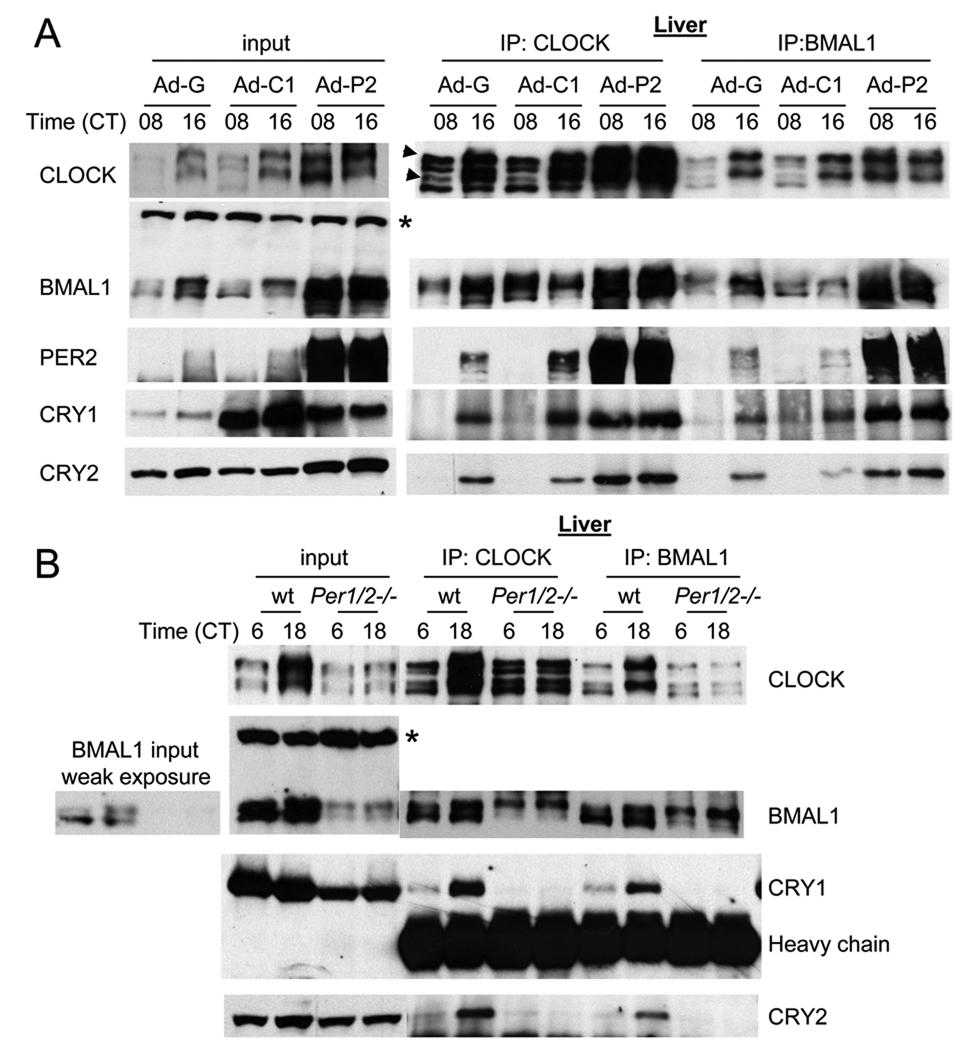
Fig. 4.
In GFP- or CRY-overexpressing liver, rhythmic interaction between CLOCK:BMAL1 and PER/CRY is maintained, but the interaction is constitutively elevated in PER2-overexpressing liver and almost absent in Per1/2ldc mutant mice. (A) Liver samples at CT 08 and 16 in Fig 4A (CD1 mice) were subjected to IP for CLOCK and BMAL1 and the resulting immunocomplexes were immunoblotted for clock proteins. Two arrow heads indicate two hyperphosphorylated isoforms of CLOCK compared to the other two isoforms as has been shown previously (Lee et al., 2001). The asterisk denotes a nonspecific band in the BMAL1 immunoblot ensuring equal loading of total protein. (B) IP for CLOCK and BMAL1 was performed using liver samples at CT06 and 18 from Per1/2ldc mutant and matching wt mice. To compensate for lower amounts of clock proteins in the mutant mice, twice as much starting material was used for the IP experiments. Note that CLOCK is hypophosphorylated, but BMAL1 is hyperphosphorylated in the mutant mice.