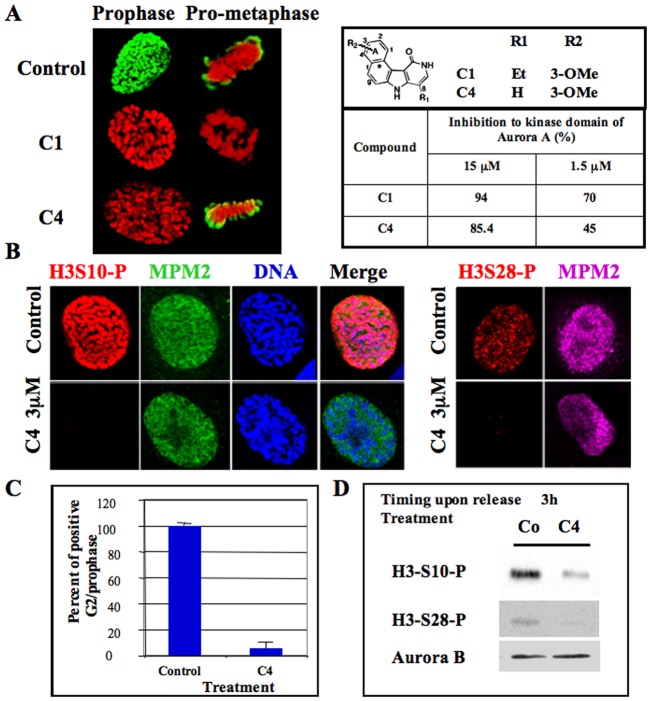
Fig. 1. Delay of histone H3 phosphorylation at mitotic entry upon C4 treatment.
(A) C1 and C4 are two benzo[e]pyridole inhibitors of the catalytic domain of aurora kinase A (Hoang et al., 2009). Molecules and their efficiency in vitro towards aurora A catalytic domain are recalled (Hoang et al., 2009). These data derived from the high throughput screening performed under non-saturating conditions. In vitro, IC50 of C1 towards aurora A, B are 61 nM and 31 nM respectively (Hoang et al., 2009). Immunofluorescences of histone H3 (Ser10) phosphorylation on HeLa cells treated by either C1 (1 µM) or C4 (3 µM) are represented; Histone H3 (Ser10) phosphorylation is shown in green whereas DNA is in red. Both prophase and pro-metaphase are imaged and compared to control cells. (B) Immunofluorescence of Histone H3 (Ser10 and Ser 28) phosphorylations on MPM2 positive HeLa cells. (C) Analysis of the percentage of positive histone H3-phospho Ser 10 at mitotic entry. Two independent experiments were conducted and 100 cells in G2/prophase scored in each. (D) Western blots were realized on cells synchronized by a MG-132 block and then released for 3 hours. Histone H3 (Ser10 and Ser 28) phosphorylations were analyzed. The same membrane was also revealed using an antibody against aurora kinase B for estimation of the amount of mitotic cells.