Abstract
A mass formed around a cotton matrix left within the body is termed as textiloma or gossypiboma. It is a rare complication of surgery most commonly seen after abdominal operations. The time of presentation may range from early post-operative period to several decades later. A correct diagnosis can be made in only one-third of the cases. The most common differential diagnosis is a new-onset or recurrent tumor. This may lead to a lot of patient anxiety as well as several unnecessary attempts at biopsy or surgery. Gossypiboma may present as either of the following syndromes - pseudotumoral, occlusive, or septic entity and the risk of fistulization increases with time. We present two diverse cases, the first case being of a patient with gastro-cutaneous fistula due to retained sponge presenting within 2 months of open cholecystectomy, while the second case presented 13 years after a hysterectomy, with abdominal lump and obstruction caused by a retained sponge.
Keywords: Gossypiboma, gastro-cutaneous fistula, intestinal obstruction
INTRODUCTION

A mass formed around a cotton matrix left within the body is termed as textiloma or gossypiboma.[1] The term “gossypiboma” is derived from the Latin word gossypium, meaning cotton, and the Swahili word boma, meaning place of concealment.[2] Since, its first report by Wilson in 1884,[1] this has been reported in 1 in 100 - 5,000 surgical interventions and 1 in 1,000 - 1,500 intra-abdominal operations.[1] Textilomas can remain silent or induce a series of inflammatory reactions though they themselves are chemically inert.[3] This reaction causes pus formation, fibrosis and/or granulomas giving rise to fistulae or pseudo-tumors.[2] Gossypibomas are difficult to diagnose because of huge variations in timing and presentation and due to the legalities involved. However, delayed or inappropriate management can lead to significant physical and psychological morbidity.
CASE REPORTS
Case 1
A 55-year-old female presented with a history of abdominal hysterectomy for fibroids, performed 13 years back. Post-operation, the patient had recurrent episodes of colicky abdominal pain and fever, without any features of obstruction. These symptoms were relieved by antibiotics. Patient also noticed a lump in her abdomen, which was migratory and remained unchanged in size over a decade. However, during the last couple of years, the lump became fixed in her lower abdomen.
At the time of the current admission the patient complained of severe colicky pain, vomiting, and constipation. Her abdominal examination revealed a 12 cm × 10 cm size well-defined, firm, non-tender, smooth, and fixed intra-abdominal lump in the right iliac fossa. There was no free fluid. Examination of other systems was essentially non-contributory. She was subsequently investigated and found to be a diabetic. Her other hematological and bio-chemical parameters were within normal limits. Her abdominal X-ray revealed an ovoid opacity, with density of soft-tissue, in the lower central abdomen along with displacement of bowel loops. There was no evidence of any radio-opaque marker. Ultrasound (USG) [Figures 1a and 1b], revealed a well-marginated, ovoid mass with echogenic walls [Figure 1a–A], and hypoechoic central cavity [Figure 1a–B]. Multiple curvilinear echogenic stripes were seen within the cavity [Figures 1a and b - C] some revealing posterior acoustic shadowing [Figure 1a - D] and some reverberation artifacts [Figure 1b - E]. On color Doppler, no flow was seen within the mass [Figure 1b].
Figure 1, Case 1.
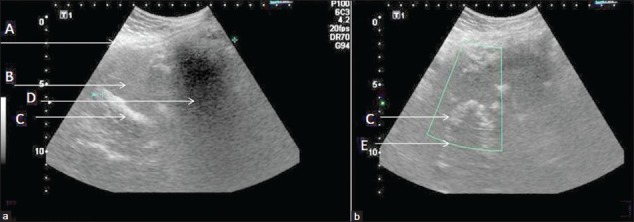
Abdominal Ultrasound and color Doppler scans of infra-umbilical Gossypiboma. (a) Transverse mid-abdominal ultrasonography image shows a well-marginated, ovoid mass with echogenic walls (A), a hypoechoic central cavity (B), and multiple curvilinear echogenic stripes within the cavity (C), some revealing posterior acoustic shadowing (D), and some, reverberation artifacts (E). (b) Longitudinal color Doppler scan through the same area, does not show any flow within the mass.
Computed tomography [Figures 2a and b] revealed a well-encapsulated ovoid mass in the umbilical region [Figure 2a-A], which was adherent to the anterior abdominal wall. It had a central cavity filled with contents showing inhomogeneous density i.e., hypodense areas interspersed with hyperdense whorled stripes, which showed no enhancement or calcification [Figure 1a - B]. Capsule was 4-5 mm thick, hyperdense, and showed moderate enhancement [Figure 1a-C]. Marked stranding was seen in surrounding omental fat especially in postero-inferior part with adherent small bowel loops - possibly ileal loops [Figure 1a-D]. There was dilatation of proximal loops and zone of transition at site of adherence, suggestive of partial obstruction [Figure 1a-E].
Figure 2, Case 1.
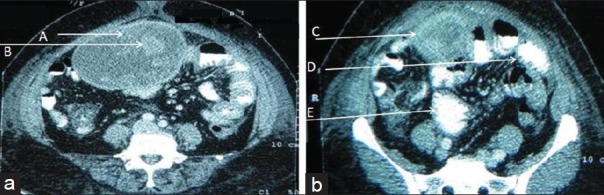
Contrast Enhanced Computed Tomography scans of the abdomen at the level of (a) umbilicus and (b) infra-umbilical region, show a well-encapsulated ovoid mass (A) with a central hypodense cavity having hyperdense whorled stripes (B). Capsule is hyperdense and shows moderate enhancement (C). Bowel loops are adherent to the mass (D) and proximal loops are dilated (E).
Magnetic Resonance Imaging (MRI) [Figures 3a and b] showed a large well-defined ovoid mass in the mid abdomen region [Figure 3a-A], adherent to anterior abdominal wall and also, to small bowel loops [Figures 3a and 3b-B]. It had a thick capsule appearing intensely hypointense on both T1-and T2-Weighted (W) images [Figure 3b-C] and a central fluid filled core with hypointense whorled stripes [Figure 3b-C]. Fluid was hyperintense to muscles in both T1-and T2 W images. All these findings were suggestive of a possibility of gossypiboma.
Figure 3, Case 1.
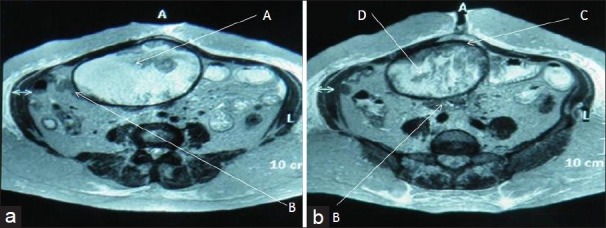
MRI abdomen-T2 W axial images of Case 1 at (a) infra-umbilical and (b) umbilical level show a large well-defined ovoid mass (A) in mid abdomen, adherent to small bowel loops (B). It has a thick hypointense capsule (C) and a central fluid filled core with whorled stripes of intermediate intensity (D).
The patient underwent exploratory laparotomy, which revealed roll gauze wrapped in thick omental cover forming a pseudo-capsule all around, with dense adhesions to surrounding small bowel [Figure 4]. Adhesionolysis was done and the gauze piece removed. The patient tolerated the surgery well and had no recurrence of symptoms.
Figure 4, Case 1.
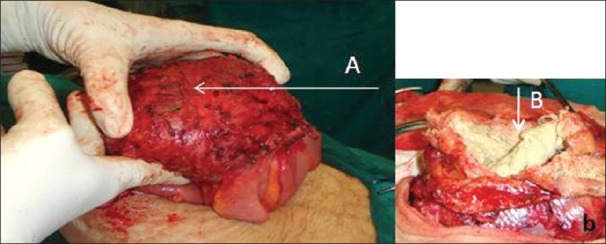
Operative photograph shows an encapsulated mass of gauze encased in omentum adherent to bowel (A). Inset shows its cut section with gauze (B) visible inside the pseudo-tumor.
Case 2
A 35-year-old female presented with a 15-day history of pus discharge and non-healing wound in the right upper abdomen along with an underlying lump. She had undergone open cholecystectomy 2 months earlier. She also experienced progressive loss of weight and appetite from a month after her surgery. There were no associated fever or bowel complaints. On physical examination, there was a 7 cm long transverse subcostal scar in the right hypochondrium with a pus discharging sinus at its lateral end. There was a fixed lump underlying the scar area. Her hematological and biochemical tests and plain skiagrams of the chest and abdomen were non-contributory. A contrast study carried out from the site of pus discharge [Figure 5a - A] demonstrated mottled pooling of contrast in right hypochondrium [Figure 5b - B]. Prompt entry of contrast into the stomach indicated a gastro-cutaneous fistula [Figure 5b - C]. On contrast enhanced CT scan, the oral contrast came out from the fistulous opening within a few minutes of ingestion, further confirming a gastro-cutaneous fistula. A well-circumscribed mass with spongiform mottled lucencies suggestive of trapped air bubbles and some oral contrast within, was seen in the right sub-hepatic space abutting the pylorus [Figure 5c and d-D]. A diagnosis of gossypiboma causing a gastro-cutaneous fistula was made.
Figure 5, Case 2.
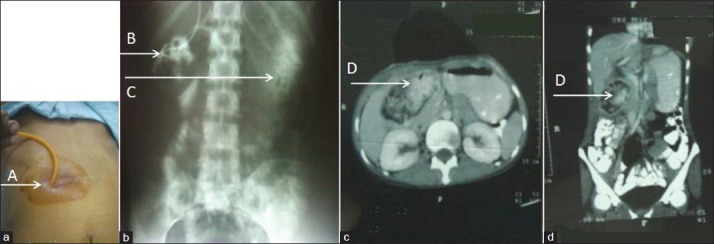
Retained sponge causing gastrocutaneous fistula. a) shows fistulogram being performed from opening at lateral end of scar b) Fistulogram performed from opening at lateral end of scar (A) shows mottled pooling of contrast in right hypochondrium (B) and contrast in stomach (C). (c) Axial CECT image at level of pylorus and (d) coronal reconstructed image through the same area show a well-circumscribed mass with contrast admixed with spongiform mottling suggestive of foreign body, in the right sub-hepatic space (D).
An exploratory laparotomy was performed. There were dense adhesions involving the stomach, transverse colon, hepatic flexure, and omentum. There was a fistulous communication with the anterior gastric wall and the presence of the sponge in the gastric cavity [Figure 6]. Adhesionolysis was carried out. The sponge was identified and removed. The necrotic anterior gastric wall was debrided and closed in layers. A feeding jejunostomy was performed to expedite enteral nutrition. Post-operative recovery was unremarkable and the jejunostomy was removed after 2 weeks.
Figure 6, Case 2.
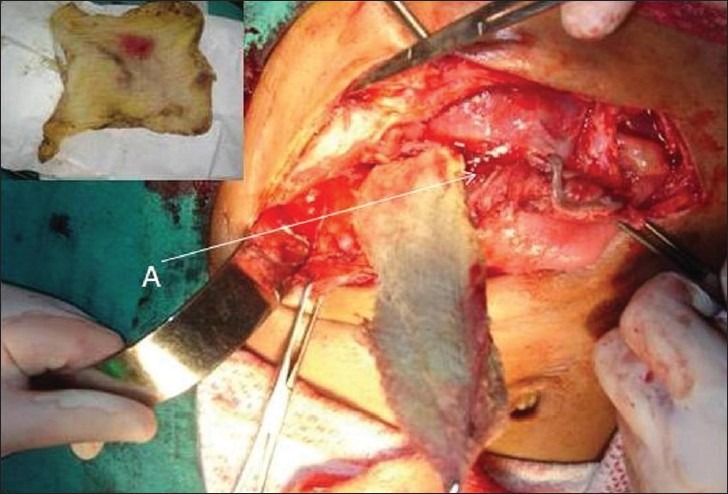
Operative photograph shows sponge being removed from gastric cavity in sub-hepatic region (A). Inset shows the removed sponge.
DISCUSSION
Gossypibomas are most frequently diagnosed in the intra-abdominal cavity[3] and abdominal sponge is the most common foreign body reported.[2] Risk factors include emergency operations and high body mass index.[1,3] A correct diagnosis of a retained swab has been reported in only one-third of all cases, with a new-onset or a recurrent tumor being the most common differential. This may lead to a lot of patient anxiety as well as unnecessary attempts at biopsy or morbid surgery.[1,4] Gossypibomas may present at any time, from a few weeks to several decades after initial surgery. They may present as either of the following syndromes - pseudotumoral, occlusive, or septic entities.[4] Patients generally complain of non-specific abdominal pain, nausea, vomiting, and abdominal distension, rectal bleeding, altered bowel habit, fever, anorexia, weight loss, malabsorption syndrome, or a palpable mass.[4] They might even present with features of severe pain due to peritonitis or obstruction or as external fistulae or non-healing infection of the surgical wound. About a third of gossypiboma patients remain asymptomatic, with the foreign body solely detected on radiography.[5]
Gossypibomas may give rise two types of reactions - exudative leading to abscess formation or aseptic fibrinous giving rise to foreign body granuloma formation.[5,6] The former usually occurs early in the post-operative period and may involve secondary bacterial contamination, which results in various fistulae.[1] The longer the retention time, the higher is the risk of fistulization.[7] Transmural migration of sponge can occur in various intra-abdominal locations. During the first 2 months of retained gauze, there is little reaction. From 2 months to 2 years, infective inflammation and abscess are found. During this period, extrusion of the gauze may occur internally or through an external fistulous tract.[8] In delayed phase, presentation may be as a mass and/or intestinal obstruction.
Radiology is the mainstay of pre-operative diagnosis of gossypiboma. Plain skiagram or a direct contrast study through a fistulous opening might show a radio-opaque marker or the sponge. Radio-opaque threads impregnated into surgical gauzes were first introduced by Cahn in 1929, but came into general use in the United States in 1940s.[8] However, in Asian countries this practice came several decades later in the 1980s.[9] The markers may be distorted by folding, twisting or disintegration over time. Thus, diagnosis is difficult using only plain X-ray.[9] No radio-opaque markers were seen in our cases also.
On ultrasonography gossypibomas may present as - (i) an echogenic area with intense posterior shadow; (ii) in cases of exudative reactions they are seen as a well-defined cystic mass containing distinct internal hyperechoic wavy, striped focus - as seen in our cases and (iii) non-specific pattern with a hypoechoic mass or a complex mass.[7] Acoustic shadowing is observed in all cases. This is due to the attenuation of beam by foreign body as well as presence of gas and sometimes calcification.[1,6]
The characteristic CT feature is a low-density heterogeneous whorl-like spongiform hypodense mass containing air bubbles with an external high-density wall that is further highlighted on contrast-enhanced imaging. Calcification of the mass wall or reticulate rind sign may be observed on CT.[1,2] Today CT is the main modality for diagnosis (61%), followed by radiography (35%), and USG (34%).[10]
Gossypiboma is best removed by open surgery even though laparoscopic and percutaneous removal has been reported in a few selected cases.
CONCLUSION
A foreign body left behind after an operation is a medico legal issue and often under-reported.[1,5] Prevention is the best management for this entirely avoidable complication and Gencosmanoglu and Inceoglu have advised: (1) meticulous count of all surgical materials, (2) thorough exploration of the surgical site at the conclusion of the procedures, and (3) routine use of surgical textile materials impregnated with a radio-opaque marker.[7] Despite its low incidence, the diagnosis should be considered in all patients presenting with unexplained symptoms, mass, or fistulae with a history of prior surgery and imaging should be carefully evaluated. CT scan remains the primary modality of pre-operative diagnosis. A shut eye response because of egos or legalities involved can lead to delay in diagnosis and management which can further complicate the patient's condition.
Footnotes
Available FREE in open access from: http://www.clinicalimagingscience.org/text.asp?2013/3/1/11/107998
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Manzella A, Filho PB, Albuquerque E, Farias F, Kaercher J. Imaging of gossypibomas: Pictorial review. AJR Am J Roentgenol. 2009;193:S94–101. doi: 10.2214/AJR.07.7132. [DOI] [PubMed] [Google Scholar]
- 2.Lu YY, Cheung YC, Ko SF, Ng SH. Calcified reticulate rind sign: A characteristic feature of gossypiboma on computed tomography. World J Gastroenterol. 2005;11:4927–9. doi: 10.3748/wjg.v11.i31.4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moyle H, Hines OJ, McFadden DW. Gossypiboma of the abdomen. Arch Surg. 1996;131:566–8. doi: 10.1001/archsurg.1996.01430170112022. [DOI] [PubMed] [Google Scholar]
- 4.Buluş H, Şımşek G, Coşkun A, Koyuncu A. Intraabdominal gossypiboma mimicking gastrointestinal stromal tumor: A case report. Turk J Gastroenterol. 2011;22:534–6. doi: 10.4318/tjg.2011.0269. [DOI] [PubMed] [Google Scholar]
- 5.Hu SC, Pang HL, Hsieh HF. Gossypiboma (retained surgical sponge): Report of a case. Gastroenterol J Taiwan. 2005;22:329–34. [Google Scholar]
- 6.Malik A, Jagmohan P. Gossypiboma: US and CT appearance. Indian J Radiol Imaging. 2002;12:503–4. [Google Scholar]
- 7.Gencosmanoglu R, Inceoglu R. An unusual cause of small bowel obstruction: Gossypiboma - Case report. BMC Surg. 2003;3:6. doi: 10.1186/1471-2482-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shyung LR, Chang WH, Lin SC, Shih SC, Kao CR, Chou SY. Report of gossypiboma from the standpoint in medicine and law. World J Gastroenterol. 2005;11:1248–9. doi: 10.3748/wjg.v11.i8.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng TC, Chou AS, Jeng CM, Chang PY, Lee CC. Computed tomography findings of gossypiboma. J Chin Med Assoc. 2007;70:565–9. doi: 10.1016/S1726-4901(08)70063-7. [DOI] [PubMed] [Google Scholar]
- 10.Mirfazaelian H, Ansari M, Daneshbod Y. Gossypiboma. Rev Assoc Med Bras. 2012;58:638. [PubMed] [Google Scholar]


