Abstract
Sapropterin, a synthetic form of tetrahydrobiopterin (BH4), has been reported to improve symptoms in children with autism spectrum disorder (ASD). However, as BH4 is involved in multiple metabolic pathway that have been found to be dysregulated in ASD, including redox, pterin, monoamine neurotransmitter, nitric oxide (NO) and immune metabolism, the metabolic pathway by which sapropterin exerts its therapeutic effect in ASD effect remains unclear. This study investigated which metabolic pathways were associated with symptomatic improvement during sapropterin treatment. Ten participants (ages 2–6 years old) with current social and/or language delays, ASD and a central BH4 concentration ⩽30 nℳ l−1 were treated with a daily morning 20 mg kg−1 dose of sapropterin for 16 weeks in an open-label fashion. At baseline, 8 weeks and 16 weeks after starting the treatment, measures of language, social function and behavior and biomarkers of redox, pterin, monoamine neurotransmitter, NO and immune metabolism were obtained. Two participants discontinued the study, one from mild adverse effects and another due to noncompliance. Overall, improvements in subscales of the Preschool Language Scale (PLS), Vineland Adaptive Behavior Scale (VABS), Aberrant Behavior Checklist (ABC) and autism symptoms questionnaire (ASQ) were seen. Significant changes in biomarkers of pterin, redox and NO were found. Improvement on several subscales of the PLS, VABS, ABC and ASQ were moderated by baseline and changes in biomarkers of NO and pterin metabolism, particularly baseline NO metabolism. These data suggest that behavioral improvement associated with daily 20 mg kg−1 sapropterin treatment may involve NO metabolism, particularly the status of pretreatment NO metabolism.
Keywords: autism, monoamine neurotransmitters, nitric oxide, sapropterin, tetrahydrobiopterin
Introduction
The autism spectrum disorders (ASD) are a heterogeneous group of neurodevelopmental disorders that are behaviorally defined by impairments in communication and social interactions along with restrictive and repetitive behaviors.1 An estimated 1 of 88 individuals in the United States is affected with ASD.2 Although several genetic syndromes are associated with ASD, these genetic syndromes only account for 6–15% of ASD cases.3 Evidence from many fields of medicine has documented that multiple non-central nervous system abnormalities are associated with ASD,4, 5, 6, 7, 8 suggesting that ASD may involve systemic abnormalities, rather than organ-specific abnormalities, at least in some individuals with ASD. Indeed, over the last decade, physiological systems that transcend specific organ dysfunction, such as immune dysregulation, inflammation, impaired detoxification, environmental toxicant exposures, redox regulation/oxidative stress and mitochondrial dysfunction, have been implicated.8, 9
Evidence-based treatments for ASD are limited. Currently, there is no food and drug administration approved medical therapy that addresses either core ASD symptoms or pathophysiological processes associated with ASD. One pharmaceutical treatment that could potentially improve core ASD symptoms as well as associated physiological abnormalities associated with ASD is sapropterin. Two controlled and several open-label studies conducted over the past 25 years have documented a favorable response to sapropterin, a synthetic form of tetrahydrobiopterin (BH4), for the treatment of children with ASD.10 Over a 6-year period, from 1985–1990, four Japanese researchers studied over 300 mildly-to-severely affected ASD children in five open-label studies and one double-blind placebo controlled study.11, 12, 13, 14, 15, 16, 17, 18, 19, 20 Oral sapropterin at a dose of 1–3 mg kg−1 per day over a 4–24-week treatment period resulted in a 41–64% response rate. Two additional studies, a double-blind placebo controlled crossover study and an open-label study, conducted by Swedish researchers using oral sapropterin at a dose of 3–6 mg kg−1 per day resulted in improvement in ASD symptom.21, 22 Overall, studies have reported improvements in communication,16, 22 cognitive ability,16, 22 adaptability,16 social abilities21, 22 and verbal expression.16 Specific patient characteristics have been associated with better treatment response. Children under 5 years of age responded better than older children in two studies,14, 23 and higher baseline intellect was positively correlated with improvement in social interactions in another study.21
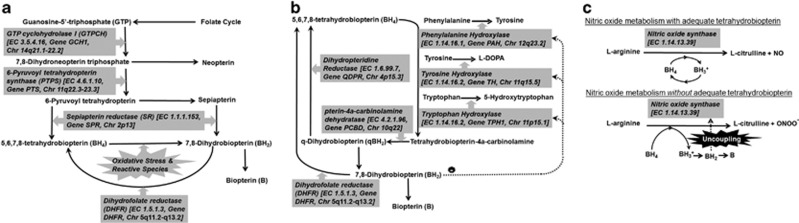
BH4 is a naturally occurring pteridine that is an essential cofactor for several critical metabolic pathways. BH4 is not obtained from the diet in any significant quantities, making pterin production essential for normal metabolism. Other pterins such as biopterin, sepiapterin and neopterin are produced by the pterin pathway, but BH4 is the primarily biologically active pterin. BH4 is synthesized de novo from guanosine-5′-triphosphate, a purine nucleotide derived from the folate cycle (Figure 1a).24
Figure 1.
Pterin metabolism. Metabolites are connected with black arrows while enzymes (with Enzyme Commission number, Gene symbol and chromosomal location) are presented in gray boxes with thick gray arrows pointing to the reaction they catalyze. Dashed lines represent inhibitory effects of metabolites on enzymes. (a) GTP cyclohydrolase I is the committing step in tetrahydrobiopterin (BH4) synthesis. 7,8-dihydroneopterin triphosphate, has one of two fates, (1) metabolism by 6-pyruvoyl tetrahydropterin synthase, the rate limiting step in BH4 production, to produce 6-pyruvoyl tetrahydropterin, or (2) shunting to produce neopterin. Sepiapterin reductase is the final step in BH4 production. Alternatively, 6-pyruvoyl tetrahydropterin can also be converted to sepiapterin which can then be metabolized to 7,8,-dihydrobiopterin (BH2). BH4 can be produced from BH2 by dihydrofolate reductase, a folate-dependent enzyme. This is one of the BH4-recycling pathways that is important in the context of oxidative stress as the interaction of BH4 with reactive species results in the production of BH2. If BH2 is not recycled, it may be nonenzymatically metabolized into biopterin. (b) BH4 is used as a cofactor for several hydroxylases that are essential for production of monoamine neurotransmitter precursors and phenylalanine degradation. These reactions result in the production of tetrahydrobiopterin-4a-carbinolamine, which can be recycled to produce BH4. In a second recycling pathway, pterin-4a-carbinolamine dehydratase metabolizes tetrahydrobiopterin-4a-carbinolamine into q-dihydrobiopterin (qBH2), which can be utilized to produce BH4 using dihydropteridine reductase. If qBH2 is not recycled, it may be nonenzymatically metabolized to BH2, which can be further metabolized into biopterin (B). (c) Under normal conditions, BH4 is not consumed during NO production but, rather, stabilizes and assists NO synthase (NOS). Initially, during L-arginine oxidation, BH4 donates electrons to the ferrous-dioxygen complex in the oxygenase domain of the enzyme, resulting in a protonated trihydrobiopterin cation radical (BH3+). BH3+ is subsequently reduced by electron transfer from NOS flavins in order to reproduce the original BH4. When BH4 availability is reduced or the reduced-to-oxidized pterin ratio is unfavorable, electron transfer from NOS flavins to BH3+ becomes uncoupled from L-arginine oxidation. This results in the generation of peroxynitrite, a reactive nitrogen species that has been linked to cell toxicity, and the production of BH2 from the unstable BH3+ radical. BH2 can further be metabolized into B and inhibits NOS activity. Thus, in the presence of low BH4 or a low reduced-to-oxidized pterin ratio, NOS activity can result in a downward spiral in which BH4 is destroyed and a peroxynitrite is produced instead of NO, BH4 availability and the reduced-to-oxidized pterin ratio is progressively lowered, and NOS if further uncoupling, resulting in the further destruction of BH4.
BH4 has several major functions. BH4 is a cofactor for hydroxylases that produce monoamine neurotransmitter precursors and degrade phenylalanine, and is critical for nitric oxide (NO) production (Figures 1b and c). Interestingly, the BH4 concentration (or the reduced-to-oxidized pterin ratio) is critical for regulating the whether BH4 is consumed during NO production. In the context of a normal BH4 concentration (or normal reduced-to-oxidized pterin ratio), BH4 is not consumed during NO production but is rather recycled locally within the reaction's microenvironment. However, low BH4 concentrations or a reduced-to-oxidized pterin ratio results in a vicious spiral, where BH4 is progressively consumed and the reaction becomes uncoupled, such that a peroxynitrite, a reactive nitrogen species that is cytotoxic, is produced instead of NO. Lastly it is important to consider that BH4 is an antioxidant (Figure 1a).
In general, sapropterin treatments studies have assumed that the therapeutic effect of BH4 is the result of enhancement of monoamine neurotransmitter metabolism, perhaps because ASD has been associated with monoamine neurotransmitters abnormalities9, 25 and depressed BH4 cerebrospinal fluid (CSF) concentrations.22, 26 Yet only two treatment studies have measured CSF BH4 concentrations,21, 22 and only one examined the relationship between CSF BH4 concentration and treatment response.21 In this latter study, the correlation between CSF BH4 concentration before treatment and social interaction improvement was only borderline significant.21 Fernell et al.22 used positron emission tomography to examine dopamine metabolism changes with BH4 treatment; a decrease in the baseline elevation in D2 receptor binding occurred with BH4 treatment but this change was not correlated with treatment response.
Given that multiple metabolic systems are influenced by BH4 metabolism, it is essential to determine which metabolic systems account for treatment response. Such information would lead to a better understanding of ASD pathophysiology and the development of biomarkers that predict response to BH4 treatment. In this study we examined biomarkers of redox, pterin, monoamine neurotransmitter, NO and immune metabolism, along with measures of language, social function and behavior, in 10 ASD participants treated with sapropterin for 16 weeks in an open-label fashion.
Materials and methods
Design overview
This study was a prospective 16-week open-label outpatient treatment trial of sapropterin for core and associated ASD symptoms in 2–6-year-old children with confirmed language and/or social delays, ASD and CSF BH4 concentration ⩽30 nℳ l−1. This CSF levels was chosen because of the fact that individuals with neurodevelopmental disorders and CSF BH4 levels above 30 nℳ l−1 have been found to have seizures or an abnormal electroencephalogram.25 This study was approved by the Institutional Review Board of the University of Texas Health Science Center at Houston (Houston, TX, USA). This clinical trial was investigator sponsored and registered at clinicaltrials.gov as NCT01141595.
Sample
Study participants were recruited from the University of Texas Medically-Based Autism Clinic by the first author. All children met the Diagnostic and Statistical Manual of Mental Disorders—Fourth Edition—Text Revision (DSM–IV–TR)1 criteria for ASD and had previously been diagnosed by a developmental pediatrician, pediatric neurologist or clinical psychologist with ASD who also ruled-out alternative diagnosis and psychiatric comorbidities. ASD was confirmed using DSM–IV–TR diagnostic criteria at the time of evaluation. Children selected for this trial had severe enough deficits that they underwent a comprehensive metabolic and neurological workup including a CSF examination because they were not improving with standard therapies. All participants had (a) significant residual language and/or social delays as determined by a Preschool Language Scale 4th Edition (PLS) Total scaled score ⩽70 and/or a parent reported Social Responsiveness Scale (SRS) scaled score ⩾76, and (b) a developmental quotient ⩾50 as assessed with the Vineland Adaptive Behavior Scales (VABS), 2nd Edition, Interview Edition, Survey Form.
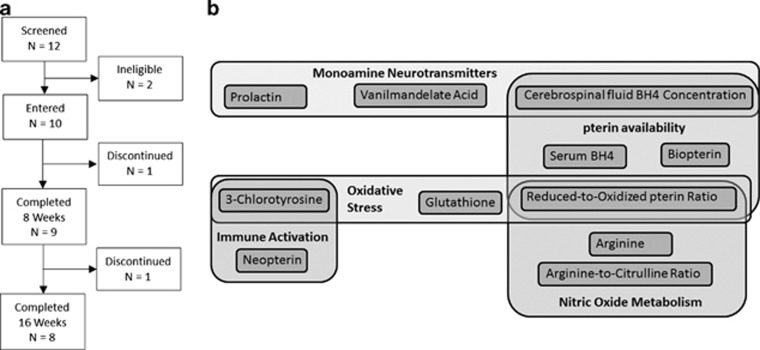
Other enrollment criteria included being between 2–6 years of age at the start of the study, having unchanged therapy in the previous 2 months and agreeing to not change any traditional or alternative medical or behavioral therapy during the study. Patients were excluded if they had epilepsy, a history of seizures, an electroencephalogram with seizure discharges, a genetic syndrome, a metabolic disorder that would contraindicate the use of BH4, prematurity, developmental delays secondary to a pure motor disorder, current gastroesophageal reflux, current or history of liver or kidney disease, severe irritability, concurrent use of dopamine drugs or drugs that effect folate or NO metabolism. Of the 12 subjects who showed initial interest to participate, two did not meet inclusion criteria. The remaining participants (n=10) entered into the study (Figure 2).
Figure 2.
(a) Recruitment and discontinuation of participants in the study; (b) Biomarkers to measures various aspects of tetrahydrobiopterin (BH4) metabolism. Biomarkers are divided into five different categories: Monoamine neurotransmitter, oxidative stress, pterin availability, immune activation and nitric oxide metabolism. Many of the biomarkers overlap into several categories.
Study medication
A measure of 20 mg kg−1 of BH4 was given once daily as 100 mg tablets of Kuvan (BioMarin Pharmaceutical, Novato, CA, USA). The child's weight was measured on inclusion; children remained on the same dose throughout the study unless the participant underwent a dosage reduction. If adverse effects occurred, parents were given the option of decreasing the dose to 10 mg kg−1 per day or discontinuing the trial. At the end of the study, parents returned unused pills. No child missed a significant number of doses. Although many previous studies have used much lower doses of sapropterin ranging from 1 to 6 mg kg−1,11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22 the current study used a higher dose, 20 mg kg−1, because this is the food and drug administration approved dose of Kuvan (BioMarin Pharmaceutical, Novato, CA, USA) in children aged 2 years and above in the United States for phenylketonuria. Thus, the safety and pharmacokinetics of this Kuvan dose has been well studied.
Procedures and measures
All cognitive evaluations were conducted or supervised by a child psychologist. At baseline and weeks 8 and 16, primary and secondary outcome measures were administered. Questionnaires required for several of the outcome measures were mailed to the parents and teachers approximately a week before the visit. Adverse effects were monitored at visits and at weeks 4 and 12 by phone.
The primary outcome measure was language skills as assessed by the PLS.27 The PLS is comprised of receptive, expressive and total communication scores. The remaining assessments were secondary outcomes. Adaptive behavior was assessed using the VABS, an instrument that has demonstrated good reliability and validity.28 Nine subscales were examined: receptive, expressive and writing communication, personal, domestic and community daily living skills, interpersonal relations, play and coping skills. For the PLS and VABS, raw scores were used to follow changes, whereas standardized scores were used for inclusion. For both the PLS and VABS, higher-scaled scores and raw scores represented better performance on these measures.
Social skills were assessed using a observational parent–child interaction scale, and parent and teacher SRS.29 The observational parent–child interaction scale was developed and validated with children born prematurely.30, 31 Parent and child spent 15 min in a room with a standardized set of toys. The session was recorded using a digital audiovisual camera. At the end of the trial, two observers blindly rated the videos on several behavioral scales (interrater reliability ranged from 0.82 to 0.98 across scales). Children were rated for responsiveness, social engagement and affect. Higher scores on the observation scales represented better performance. The SRS produces a raw score and a T-score; higher raw or T-scores on the SRS indicate worse social function. The SRS has a high correlation with the autism diagnostic observation schedule (ADOS), which is the goal standard for the diagnosis of ASD.32
Behavior was assessed using the parent and teacher Aberrant Behavior Checklist (ABC).33 Subscales represent irritability, social withdrawal, stereotypic behaviors, hyperactivity and inappropriate speech. Autism symptoms were assessed using the autism symptoms questionnaire (ASQ), a DSM–IV–TR-based checklist developed by The Center for Autism and Related Disorders (Tarzana, CA, USA). The ASQ generates a score from 0 to 4 for the social interaction and stereotyped behavior scales and a score from 0 to 5 for the communication scale. Raw scores are used for both the ABC and ASQ; higher scores represent worse behavior.
Analytical approach
Mixed-effects regression models34 were conducted via SAS version 9.3 (SAS Institute Inc., Cary, NC, USA) ‘glmmix' procedure. The first model included a linear effect of time on raw primary and secondary outcome measure scores as well as biomarkers. A random intercept was used to account for each individual's symptom level. In general, two-tailed α=0.05 was used.
Next we determined whether biomarkers moderated changes in outcome variables. We conducted two sets of analyses; one that examined the baseline biomarker value and another that examined the change in biomarker value over the treatment period. As there are 10 biomarker variables (not counting the baseline CSF BH4 levels) the α-value was Bonferroni corrected to 0.005. Only the interactions between biomarker and the change in the outcome variable are reported. Significant relationships between biomarkers and outcome variables were investigated further using Pearson correlations.
Finally, the eight participants that completed the trial were divided into responders and nonresponders based on their performance on the primary outcome variable. For each biomarker, the change in its value across the treatment period was compared between the responders and nonresponders. Mixed-model regression was used as described above with a categorical variable representing the two groups. Responders and nonresponders were matched on age.
Biomarkers
Biomarkers were selected to represent a comprehensive overview of the metabolic processes associated with BH4 (Figure 2b). Monoamine neurotransmitters were monitored using serum prolactin, a reflection of central dopamine metabolism and urine vanilmandelate acid, an end-stage metabolite of norepinephrine metabolism. Pterin metabolism was monitored by serum BH4 and biopterin (B) and the reduced-to-oxidized pterin (BH4/B+BH2) ratio. The baseline CSF BH4 level was believed to reflect the initial central BH4 concentration and baseline monoamine neurotransmitter metabolism. NO metabolism was monitored by the reduced-to-oxidized pterin ratio, L-arginine and L-arginine-to-L-citrulline ratio. Oxidative stress was monitored by the free reduced-to-oxidized glutathione ratio (GSH/GSSG) and 3-Chlorotyrosine (3CT), a measure of reactive nitrogen species and myeloperoxidase activity. Immune system activation was monitored by neopterin as well as 3CT.
Biomarkers collection and processing
CSF was obtained by lumbar puncture under general sedation. CSF demonstrated a normal number of white and red blood cells, protein and glucose concentrations. CSF was collected with standardized reagent tubes and frozen at −80 °C. BH4 concentration was measured by reversed-phase high performance liquid chromatography (HPLC) with electrochemical detection35 by Medical Neurogenetics (Atlanta, GA, USA).
All biomarkers were collected as morning fasting samples before medication administration. For oxidative stress biomarkers, 4 ml of blood was collected into an EDTA-Vacutainer tube, chilled on ice and centrifuged at 4000 g for 10 min at 4 °C. Plasma was stored at −80 °C and underwent HPLC with electrochemical detection36 within 2 weeks. For prolactin, 1 ml of serum was collected in a Vacutainer without additives and analyzed using ADVIA Centaur immunochemiluminometric assay (Siemens Healthcare Diagnostics Inc., Deerfield, IL, USA). Vanilmandelate acid was analyzed using gas chromatography/mass spectrometry. For the amino acids, 4 ml of blood was collected in a sodium heparin Vacutainer and analyzed using liquid chromatography/mass spectrometry. Amino acids, vanilmandelate acid and prolactin analyses were performed by Quest Diagnostics Nichosls Institute (San Juan Capistrano, CA, USA). For neopterin, 1 ml of serum was collected in a covered Vacutainer without additives, refrigerated and measured using enzyme immunoassay by Labcorp (Houston, TX, USA). Pterins were measured using an assay developed by the BioAnalytical Sciences department at BioMarin Pharmaceutical. 2 ml of blood was collected in an EDTA-Vacutainer tube, placed on wet ice, centrifuged for 15 min at 2000–3000 RCF at 4 °C, divided into two 500-μl aliquots, transferred into cryo tubes containing 0.1% dithioerythritol and frozen at −80 °C until analysis. BH4, dihydrobiopterin (BH2) and B were measured using reverse phase HPLC for separation followed by electrochemical and fluorescence detection.
Results
Patient characteristics
On average, participants were 60 months (s.d.=13.9), 90% male, 90% Caucasian and 10% African–American. Eighty percent of participants met criteria for autistic disorder, whereas 20% met criteria for pervasive developmental disorder-not otherwise specified. On average, CSF BH4 concentration was 21.2 (s.d.=5.3) nmol l−1.
Cognitive and behavior changes over the treatment period
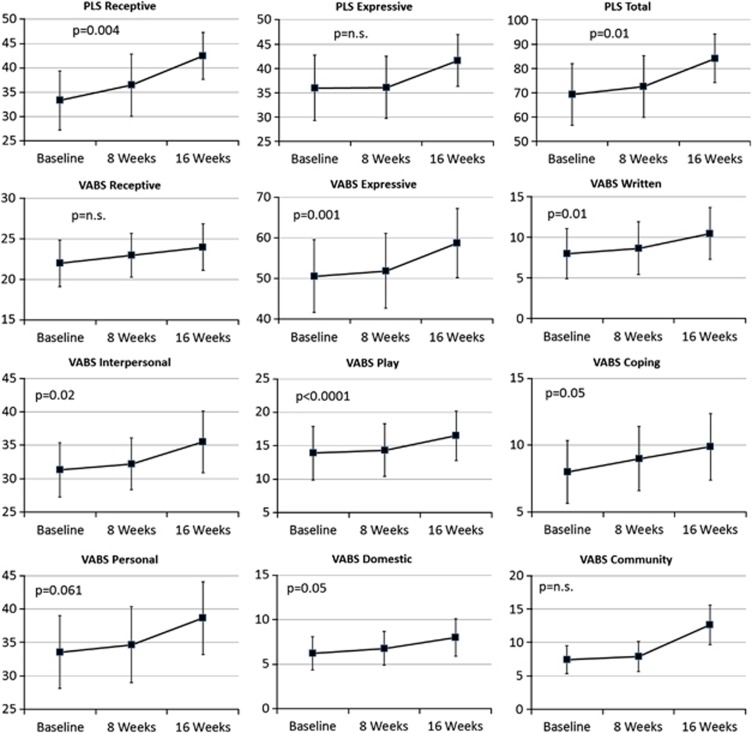
Scores for the PLS and VABS are depicted in Figure 3, whereas other scores for the other secondary measures are provided in Table 1.
Figure 3.
Change in the Preschool Language Scale 4th Edition (PLS) and Vineland Adaptive Behavior Scales (VABS), 2nd Edition, Interview Edition, Survey Form raw scores across the 16-week treatment period. P-values represent the significant of the change in the specific score over the treatment period.
Table 1. Descriptive statistics for questionnaires and parent–child interaction scales.
| Baseline | 8-weeks | 16-weeks | |
|---|---|---|---|
| M (s.d.) | M (s.d.) | M (s.d.) | |
| N=9 | N=9 | N=8 | |
| Aberrant behavior checklist—parent | |||
| Irritability | 16.3 (10.6) | 12.0 (8.4) | 11.3 (6.7) |
| Social withdrawal/lethargy | 15.3 (10.7) | 11.4 (10.2) | 8.8 (6.6) |
| Stereotypy | 7.8 (7.5) | 7.3 (6.5) | 4.9 (3.1) |
| Hyperactivity | 24.8 (11.2) | 18.5 (12.3) | 14.8 (6.7) |
| Inappropriate speech | 3.6 (3.5) | 1.9 (2.7) | 2.8 (2.3) |
| Aberrant behavior checklist—teacher | |||
| Irritability | 14.6 (11.3) | 12.6 (10.3) | 12.7 (11.7) |
| Social withdrawal/lethargy | 10.6 (8.3) | 12.9 (10.4) | 7.7 (6.5) |
| Stereotypy | 5.3 (6.4) | 5.9 (6.4) | 4.1 (3.4) |
| Hyperactivity | 16.5 (12.2) | 18.0 (11.9) | 13.9 (7.6) |
| Inappropriate speech | 1.8 (2.4) | 3.8 (4.1) | 2.6 (2.7) |
| Social responsiveness scale—parent | |||
| Awareness | 14.6 (6.3) | 13.6 (4.5) | 11.9 (3.8) |
| Cognition | 17.4 (8.5) | 17.8 (8.1) | 16.5 (5.9) |
| Communication | 33.1 (17.0) | 32.9 (13.6) | 28.0 (7.9) |
| Motivation | 13.6 (7.6) | 13.6 (7.9) | 11.8 (4.3) |
| Mannerisms | 15.4 (9.7) | 15.0 (7.3) | 13.3 (4.2) |
| Social responsiveness scale—teacher | |||
| Awareness | 12.3 (4.1) | 11.5 (4.8) | 9.2 (5.3) |
| Cognition | 17.1 (6.6) | 17.3 (5.3) | 14.2 (6.4) |
| Communication | 28.7 (14.8) | 31.7 (17.3) | 21.8 (14.3) |
| Motivation | 14.1 (7.2) | 14.7 (7.3) | 12.8 (8.0) |
| Mannerisms | 14.0 (9.0) | 15.2 (10.5) | 11.0 (10.0) |
| Autism symptoms questionnaire—parent | |||
| Social | 3.0 (1.6) | 2.6 (1.4) | 1.9 (1.7) |
| Communication | 4.1 (1.5) | 3.9 (1.6) | 2.9 (2.2) |
| Stereotypy | 2.8 (1.4) | 3.1 (2.1) | 2.5 (1.4) |
| Total | 9.9 (2.2) | 9.6 (1.4) | 7.3 (3.7) |
| Parent–child interaction scale | |||
| Social engagement | 3.3 (1.7) | 3.1 (1.5) | 3.7 (1.4) |
| Social smile | 2.8 (2.0) | 2.6 (1.9) | 2.6 (2.0) |
| Eye contact | 3.8 (2.2) | 3.7 (2.4) | 3.3 (2.5) |
| Cooperation | 3.6 (1.6) | 3.3 (1.4) | 4.2 (1.2) |
| Communication: words | 3.2 (1.6) | 3.2 (1.6) | 3.8 (1.6) |
| Communication: gestures—parent initiated | 4.0 (1.7) | 3.7 (1.2) | 4.0 (1.8) |
| Communication: gestures—child initiated | 2.9 (1.7) | 2.6 (1.7) | 2.9 (1.7) |
| Redirection | 5.3 (0.7) | 5.4 (0.5) | 5.4 (0.5) |
| Approach | 3.6 (1.6) | 3.6 (1.5) | 4.0 (1.1) |
| Stereotypical behavior | 4.1 (1.9) | 4.0 (1.4) | 4.3 (0.9) |
| Functional play | 3.9 (2.3) | 3.6 (1.8) | 4.4 (2.1) |
Significant improvements occurred in PLS receptive (F(1,16)=11.75, P<0.01) and total (F(1,16)=8.52, P=0.01) language scales as manifested as increases in PLS raw scores. Significant improvements occurred in the VABS expressive (F(1,16)=14.94, P=0.001) and writing (F(1,16)=7.58, P=0.01) communication subscales, personal (F(1,16)=32.67, P<0.0001) and domestic (F(1,16)=4.53, P=0.05) daily living skills subscales, and interpersonal relation (F(1,16)=6.88, P=0.02) and coping (F(1,16)=4.4.7, P=0.05) subscales as manifested as increases in the VABS raw scores.
Observed parent–child interaction and parent and teacher SRS scale did not significantly change. Significant improvements were found in irritability (F(1,14)=9.14, P<0.01) and hyperactivity (F(1,14)=10.85, P=0.005) subscales of the parent ABC as manifested by decreases in the raw scores. Significant improvements was found in irritability (F(1,14)=5.40, P<0.05) subscale of the teacher ABC as manifested by decreases in the raw scores. Significant improvements were found in the social (F(1,15)=4.59, P=0.05) and communication (F(1,15)=4.26, P=0.05) subscales of the ASQ as manifested by decreases in the raw scores.
Biomarker changes over the treatment period
Neither neurotransmitter biomarker significantly changed. For oxidative stress, significant increase and decrease occurred for the reduced-to-oxidized glutathione ratio (F(1,16)=14.37, P=0.001) and 3CT (F(1,16)=14.15, P=0.002), respectively. For NO metabolism, neither arginine nor the arginine-to-citrulline ratio changed significantly. For pterins, the reduced-to-oxidized pterin ratio (F(1,16)=9.24, P=0.008) significantly increased, whereas biopterin (F(1,16)=18.70, P=0.0005) significantly decreased, but BH4 did not significantly change. For inflammation, 3CT decreased significantly but neopterin did not.
Moderation of treatment effect by baseline biomarker values
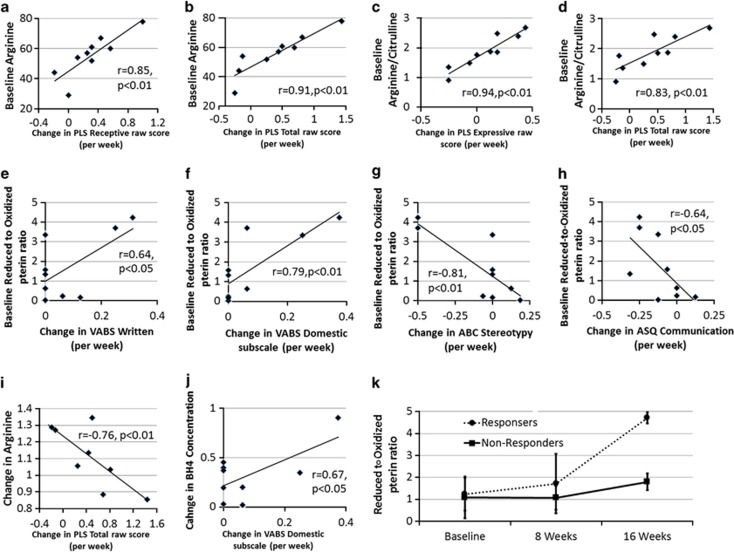
The baseline value of several NO-related biomarkers moderated changes in the primary and secondary outcome variables (Figures 4a–h). Receptive (F(1,15)=15.0, P=0.001) and total (F(1,15)=17.56, P<0.001) PLS scores increased (that is, improved) more for participants with higher baseline arginine, whereas expressive [F(1,15)=11.32, P<0.005] and total [F(1,15)=10.08, P=0.005] PLS scores increased (that is, improved) more for participants with higher baseline arginine-to-citrulline ratio. For the VABS, written (F(1,15)=12.20, P=0.003) and domestic (F(1,15)=17.15, P<0.001) subscales increased (that is, improved) more for participants with a higher baseline reduced-to-oxidized pterin ratio. Parental ABC stereotypy (F(1,13)=16.55, P=0.001) and ASQ communication (F(1,14)=9.67, P=0.008) subscales decreased (that is, improved) more for participants with higher baseline reduced-to-oxidized pterin ratios.
Figure 4.
Significant relationships between outcome variables and biomarkers. Many of the relationships found included relationships between biomarkers of nitric oxide metabolism and primary and secondary outcome variables. The relationships presented were identified using linear regression models. Pearson correlations were used to investigate the identified relationships between the outcome variable and the biomarker. P-values represent one-tailed statistical test as the direction of the relationships were predicted. Change values were calculated in change in value per week of treatment. (a–h) Relationships between outcome variables and baseline biomarker values. (i, j) Relationships between outcome variables and changes in biomarkers over the treatment period. (k) Changes in two nitric oxide metabolism biomarkers over the treatment period for four responders and four nonresponders. From these graphs it is clear that responders and nonresponders demonstrated different profiles of changes in nitric oxide metabolism.
Moderation of treatment effect by changes in biomarker over the treatment period
Several biomarkers related to NO metabolism moderated changes in the primary and secondary outcome variables (Figures 4i and j). PLS total language increased (that is, improved) more for participants with an attenuated increase in arginine over the treatment period (F(1,14)=11.39, P<0.005). VABS domestic subscale increased (that is, improved) more for participants with greater increases in BH4 over the treatment period (F(1,14)=13.88, P<0.005).
Differences in biomarkers between responders and nonresponders
Responders were found to have a significantly higher reduced-to-oxidized pterin ratio than nonresponders (F(1,18)=10.20, P=0.005) and a greater increase in the reduced-to-oxidized pterin ratio over the treatment period (F(1,18)=9.01, P<0.01) (Figure 4k).
Adverse effects
No adverse events were reported for 80% of participants. Reported adverse events were as follows: irritability, excitement and mild upset stomach (10%), and insomnia (10%). One patient underwent a dosage reduction and eventually discontinued the study. Another patient underwent a dosage reduction due to mild insomnia and remained on the decreased dose until the end of the trial.
Discussion
This study investigated the cognitive, behavioral and metabolic changes that occurred during 16 weeks of once daily 20 mg kg−1 treatment of Kuvan in 10 patients with ASD in an open-label fashion. The treatment was well tolerated with only one patient discontinuing the medication because of mild adverse effects. Significant changes in the primary and secondary outcomes and metabolic biomarkers were found, particularly for biomarkers related to NO and pterin metabolism. Most significantly, primary and secondary outcomes were moderated by metabolic biomarkers, and participants who had relatively greater improvement demonstrated differences in biomarkers values as compared with participants who had relatively lesser improvement. These analyses suggested that NO and BH4 metabolism at the beginning and during the study was related to treatment response. Overall, this study supports the notion that BH4 treatment can be effective for treating cognitive, behavioral and metabolic abnormalities associated with ASD, and that metabolic characteristics of individuals with ASD are related to their responsiveness to BH4 supplementation.
Primary and secondary outcome measures
Significant improvement was found in the primary outcome measure of language over the 16-weeks treatment period. Secondary measures, specifically VABS communication subscales and communication symptoms from the ASQ, also demonstrated improvements. This is consistent with open-label studies that showed improvements in communication,16, 22 language15, 17 and verbal expression.16 Adaptive and social behaviors also improved over the treatment period. Personal and domestic daily living skills, interpersonal and coping socialization skills of the VABS and social symptoms on the ASQ significantly improved over the treatment period. This is consistent with open-label studies that demonstrated improvement in adaptability,16 and both open-label and double-blind placebo controlled studies that demonstrated improvement in social abilities.15, 17, 21, 22 Some aberrant and ASD behaviors demonstrated significant changes over the treatment period. Both irritability and hyperactivity subscales of the ABC significantly improved over the treatment period. Double-blind placebo controlled and open-label studies have both demonstrated improvement in ASD symptom11, 12, 13, 14, 19, 20, 21, 22, 23 with BH4 treatment.
Metabolic changes over the treatment period
Interesting dynamics were observed in pterin metabolism over the treatment period. A marked increase in the reduced-to-oxidized pterin ratio along with a marked decreased in B was observed despite no significant change in BH4. This suggests that a fundamental change in pterin metabolism occurred with BH4 supplementation, as a decrease in B was not necessarily expected. A decrease in B suggests a decrease in BH2 which most likely occurred through changes in NO metabolism. Indeed, BH4 supplementation increases the reduced-to-oxidized pterin ratio and restores coupling of NO synthase (NOS) and the recycling of the trihydrobiopterin cation radical (BH3+) to BH4. This, in turn, reduces the degradation of BH3+ to BH2 and prevents the downward spiral that results in the destruction of BH4. The decrease in 3CT, a marker of reactive nitrogen species, and the increase in the reduced-to-oxidized glutathione ratio, a measure of redox state, supports this notion.
The relation between biomarkers and cognitive-behavioral changes
Greater improvement in the primary outcome measures were related to higher baseline arginine and arginine-to-citrulline ratio, and greater improvement in secondary outcome measures were related to higher baseline reduced-to-oxidized pterin ratio. Higher baseline levels of arginine, arginine-to-citrulline and reduced-to-oxidized pterin ratio all suggest better baseline NO metabolism. It is very possible that BH4 treatment improves NO metabolism, but that those patients with more dysfunctional NO metabolism require more time or higher doses of BH4 than that used in this study. This notion is supported by the fact that serum BH4, arginine and the arginine-to-citrulline ratio did not change significantly for the overall group over time. That is, only some of the participants were able to significantly change their NO metabolism with the dose of Kuvan used in this study.
NO metabolism and ASD
Consistent with our findings, several studies have documented alternations in NO metabolism in children with ASD. Two studies have associated ASD with alterations in genes that encode NOS, the enzymes responsible for NO production.37, 38 Several studies have shown that children with ASD have increased blood levels of NO as compared with age-matched controls.39, 40, 41, 42 At the same time, increased levels of nitrotyrosine, a reactive nitrogen species that can be produced by the uncoupled NOS reaction, has been shown to be increased in the blood,43, 44 hair and nails42 and brain45, 46 of children with ASD as compared with age-matched controls. This is consistent with the notion that the NOS reaction is significantly uncoupled in children with ASD. Furthermore, two studies have demonstrated that higher concentrations of NO and its metabolites were associated with increased levels of interferon-γ in children with autistic disorder, suggesting a link between increased NO metabolism and immune activation.47
The reason for changes in NO metabolism in ASD is not clear. NO is essential for central nervous system function, where it mediates such physiological processes as neurotransmitter release, neuronal excitability, long-term potentiation and neurovascular coupling.48 NO is produced by activated immune cells, particularly microglia, and has a role in immune-mediated neurotoxicity.49 NO is known to inhibit mitochondrial function, particularly respiratory chain activity,50 and has a disproportionally greater effect on reducing the mitochondrial membrane potential in ASD lymphoblastic cell lines as compared with control lymphoblastic cell lines.51 The relationship between an increase in dysregulated NO metabolism and a decrease in BH4 availability was suggested by our earlier work, where we showed that higher concentrations of serum citruline, the product of NOS, was related to lower CSF BH4 concentrations, presumably reflecting that a central BH4 deficit was related to an increase in dysregulated NO metabolism.25 This evidence combined with the fact that participants in the current study who had markers of more favorable NO metabolism demonstrated greater response to the intervention, suggests that the increase in NO metabolism seen in some individuals with ASD is associated with greater morbidity and a less favorable prognosis.
Strengths and weaknesses of the study
The number of participants in this study was limited because of the selective population chosen to study (children that had a known CSF BH4 level) and the complicated and expensive nature of biomarkers studied. Thus, we did not include a placebo group or perform a blinded study. With the open-label design of this study, it is not clear how much improvement in skills would have occurred without treatment or with placebo. All participants in this study were not making progress with standard therapy, necessitating an extensive metabolic workup, including a lumbar puncture for CSF examination before entering this study. Thus, we look toward other double-blind placebo controlled studies to address efficacy. Despite the limitations, the behavior and cognitive findings of this study are consistent with previous treatment studies in Japan and Sweden and with the recent double-blind placebo control study completed at the Children's Health Council in Palo Alto, California, USA.52
Conclusions
This study extends our understanding of the effect of BH4 treatment on the cognitive and behavioral symptoms of individuals with ASD, and suggests that the response to BH4 treatment may primarily involve NO and BH4 metabolism and that biomarkers of NO metabolism before treatment may be helpful in predicting the response to BH4 supplementation. In addition, these data provide insight into the nature of the physiology of nonresponders and helps clarify why they may not respond to the current BH4 treatment regimen.
Acknowledgments
This research was funded by BioMarin Pharmaceutical, as an investigator initiated study. Study drug was provided by BioMarin Pharmaceutical.
Dr Frye received funding for this trial by Biomarin Pharmaceutical, the manufacture of Kuvan, the form of sapropterin used in this study. The remaining authors declare no conflict of interest.
References
- APA Diagnostic and statistical manual of mental disorders4th edn.American Psychiatric Association: Washington, DC, USA; 1994 [Google Scholar]
- Rice C. Prevalence of autism spectrum disorders—Autism and Developmental Disabilities Monitoring Network, United States, 2006. MMWR Surveill Summ. 2009;58:1–20. [PubMed] [Google Scholar]
- Schaefer GB, Mendelsohn NJ. Genetics evaluation for the etiologic diagnosis of autism spectrum disorders. Genet Med. 2008;10:4–12. doi: 10.1097/GIM.0b013e31815efdd7. [DOI] [PubMed] [Google Scholar]
- Rossignol DA, Frye RE. Mitochondrial dysfunction in autism spectrum disorders: a systematic review and meta-analysis. Mol Psychiatry. 2011;17:290–314. doi: 10.1038/mp.2010.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buie T, Campbell DB, Fuchs GJ, Furuta GT, Levy J, Vandewater J, et al. Evaluation, diagnosis, and treatment of gastrointestinal disorders in individuals with ASDs: a consensus report. Pediatrics. 2010;125 (Suppl 1:S1–18. doi: 10.1542/peds.2009-1878C. [DOI] [PubMed] [Google Scholar]
- James SJ, Melnyk S, Jernigan S, Cleves MA, Halsted CH, Wong DH, et al. Metabolic endophenotype and related genotypes are associated with oxidative stress in children with autism. Am J Med Genet B Neuropsychiatr Genet. 2006;141:947–956. doi: 10.1002/ajmg.b.30366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Pessah I, Van de Water J. Elevated plasma cytokines in autism spectrum disorders provide evidence of immune dysfunction and are associated with impaired behavioral outcome. Brain Behav Immun. 2010;25:40–45. doi: 10.1016/j.bbi.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossignol DA, Frye RE. A review of research trends in physiological abnormalities in autism spectrum disorders: immune dysregulation, inflammation, oxidative stress, mitochondrial dysfunction and environmental toxicant exposures. Mol Psychiatry. 2012;17:389–401. doi: 10.1038/mp.2011.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming X, Brimacombe M, Chaaban J, Zimmerman-Bier B, Wagner GC. Autism spectrum disorders: concurrent clinical disorders. J Child Neurol. 2008;23:6–13. doi: 10.1177/0883073807307102. [DOI] [PubMed] [Google Scholar]
- Frye RE, Huffman LC, Elliott GR. Tetrahydrobiopterin as a novel therapeutic intervention for autism. Neurotherapeutics. 2010;7:241–249. doi: 10.1016/j.nurt.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naruse H, Hayashi T, Takesada M, Nakane A, Yamazaki K. [Metabolic changes in aromatic amino acids and monoamines in infantile autism and development of new treatment related to the finding] No to hattatsu. 1989;21:181–189. [PubMed] [Google Scholar]
- Naruse H, Hayashi T, Takesada M, Nakane A, Yamazaki K. Therapeutic effect of tetrahydrobiopterin in infantile autism. Proc Jpn Acad. 1987;63B:231–233. [Google Scholar]
- Naruse H, Takesada M, Nagahata M, Kazamatsuri H, Nakane Y, Yamazaki K. An open clinical study of sapropterin hydocloride (R-tetrahydrobiopterin SUN 588) in infantile autism: clinical study using a Rating Scale for Abnormal Behaviors in Children. Rinsho Iyaku. 1990;6:1343–1368. [Google Scholar]
- Naruse H, Takesada M, Nagahata M, Kazamatsuri H, Nakane Y, Yamazaki K. An open clinical study of sapropterin hydrochloride (R-tetrahydrobiopterin SUN 0588) in infantile autism - clinical study using a Rating Scale for Abnormal Behaviors in Children. Rinsho Iyaku. 1990;6:1859–1875. [Google Scholar]
- Nagahata M, Kazamatsuri H, Naruse H, Yamazaki K, Takesada M, Nakane Y, et al. Clinical evaluation of aproterin hydrochloride (R-THBP. SUN 0588) on infantile autism—a multicenter cooperative study. Rinsho Iyaku. 1990;6:1877–1899. [Google Scholar]
- Nakane Y, Asuo T, Shimogawa S, Fujiwara T, Kawabata Y, Kubota J. Clinical efficacy and effects on physical development of long-term treatment of R-tetrahydrobiopterin (R-THBP, SUN 0588) for autism. Kiso to Rinshou. 1990;24:4579–4598. [Google Scholar]
- Takesada M, Naruse H, Nagahata M.An open clinical study of aprpterin hydrochloride (R-tetrahydrobiopterin, R-THBP) in infantile autism - clinical effects and long-term follow-up International Symposium on Neurobiology of Infantile Autism: Tokyo, Japan1990
- Nakan Y, Naruse H, Hayashi T, Takesada M, Yamazaki K.Clinical effect of R-THBP on Infantile AutismIn: Naruse H, Ornitz E, (eds)Neurobiology of Infantile Autism Elsevier Science Publishers: New York, NY, USA; 337–349.1992 [Google Scholar]
- Naruse H, Hayashi T, Takesada M.A preliminary study on clinical effect of tetrahydrobiopterin in infantile autism. Ministry of Health and Welfare; 1985.
- Naruse H, Takesada M, Nakane Y, Yamazaki K, Uchiyama T, Kaihara S, et al. Clinical evaluation of R-tetrahydrobiopterin (SUN 0588) on infantile autism — a double-blind comparative study using placebo as a control. Rinsho Iyaku. 1990;6:1343–1368. [Google Scholar]
- Danfors T, von Knorring AL, Hartvig P, Langstrom B, Moulder R, Stromberg B, et al. Tetrahydrobiopterin in the treatment of children with autistic disorder: a double-blind placebo-controlled crossover study. J Clin Psychopharmacol. 2005;25:485–489. doi: 10.1097/01.jcp.0000177667.35016.e9. [DOI] [PubMed] [Google Scholar]
- Fernell E, Watanabe Y, Adolfsson I, Tani Y, Bergstrom M, Hartvig P, et al. Possible effects of tetrahydrobiopterin treatment in six children with autism—clinical and positron emission tomography data: a pilot study. Dev Med Child Neurol. 1997;39:313–318. doi: 10.1111/j.1469-8749.1997.tb07437.x. [DOI] [PubMed] [Google Scholar]
- Naruse H, Hayahi I, Takesada M, Nakane Y, Yamazaki K, Noguchi T, et al. Therapeutic effect of tetrahydrobiopterin in infantile autism. Proc Jpn Acad. 1987;63:231–233. [Google Scholar]
- Thony B, Auerbach G, Blau N. Tetrahydrobiopterin biosynthesis, regeneration and functions. Biochem J. 2000;347 (Pt 1:1–16. [PMC free article] [PubMed] [Google Scholar]
- Frye RE. Central tetrahydrobiopterin concentration in neurodevelopmental disorders. Front Neurosci. 2010;4:52. doi: 10.3389/fnins.2010.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tani Y, Fernell E, Watanabe Y, Kanai T, Langstrom B. Decrease in 6R-5,6,7,8-tetrahydrobiopterin content in cerebrospinal fluid of autistic patients. Neuroscience Lett. 1994;181:169–172. doi: 10.1016/0304-3940(94)90586-x. [DOI] [PubMed] [Google Scholar]
- Zimmerman IL, Steiner VG, Pond RE.Preschool Language ScaleFourth EditionHarcourt Assessment: San Antonio, TX, USA; 2002 [Google Scholar]
- Sparrow S, Cicchetti D, Balla D.Vineland Adaptive Behavior Scales2nd edn.Pearson Assessments: Bloomington, MN, USA; 2005 [Google Scholar]
- Constantino JN. The Social Responsiveness Scale. Western Psychological Services: Los Angeles, CA, USA; 2002. [Google Scholar]
- Landry SH, Denson SE, Swank PR. Effects of medical risk and socioeconomic status on the rate of change in cognitive and social development for low birth weight children. J Clin Exp Neuropsychol. 1997;19:261–274. doi: 10.1080/01688639708403856. [DOI] [PubMed] [Google Scholar]
- Landry SH, Smith KE, Swank PR, Guttentag C. A responsive parenting intervention: the optimal timing across early childhood for impacting maternal behaviors and child outcomes. Dev Psychol. 2008;44:1335–1353. doi: 10.1037/a0013030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolte S, Westerwald E, Holtmann M, Freitag C, Poustka F. Autistic traits and autism spectrum disorders: the clinical validity of two measures presuming a continuum of social communication skills. J Autism Dev Disorders. 2011;41:66–72. doi: 10.1007/s10803-010-1024-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aman MG, Singh GK, Stewart AW, Field CJ. The aberrant behavior checklist: a behavior rating scale for the assessment of treatment effects. Am J Mental Deficits. 1985;89:485–491. [PubMed] [Google Scholar]
- Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- Howells DW, Hyland K. Direct analysis of tetrahydrobiopterin in cerebrospinal fluid by high-performance liquid chromatography with redox electrochemistry: prevention of autoxidation during storage and analysis. Clinica Chimica Acta; Int J Clin Chem. 1987;167:23–30. doi: 10.1016/0009-8981(87)90081-7. [DOI] [PubMed] [Google Scholar]
- Melnyk S, Pogribna M, Pogribny I, Hine RJ, James SJ. A new HPLC method for the simultaneous determination of oxidized and reduced plasma aminothiols using coulometric electrochemical detection. J Nutr Biochem. 1999;10:490–497. doi: 10.1016/s0955-2863(99)00033-9. [DOI] [PubMed] [Google Scholar]
- Delorme R, Betancur C, Scheid I, Anckarsater H, Chaste P, Jamain S, et al. Mutation screening of NOS1AP gene in a large sample of psychiatric patients and controls. BMC Med Genet. 2010;11:108. doi: 10.1186/1471-2350-11-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HW, Cho SC, Kim JW, Cho IH, Kim SA, Park M, et al. Family-based association study between NOS-I and -IIA polymorphisms and autism spectrum disorders in Korean trios. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:300–306. doi: 10.1002/ajmg.b.30798. [DOI] [PubMed] [Google Scholar]
- Sogut S, Zoroglu SS, Ozyurt H, Yilmaz HR, Ozugurlu F, Sivasli E, et al. Changes in nitric oxide levels and antioxidant enzyme activities may have a role in the pathophysiological mechanisms involved in autism. Clinica Chimica Acta; Int J Clin Chem. 2003;331:111–117. doi: 10.1016/s0009-8981(03)00119-0. [DOI] [PubMed] [Google Scholar]
- Tostes MH, Teixeira HC, Gattaz WF, Brandao MA, Raposo NR. Altered neurotrophin, neuropeptide, cytokines and nitric oxide levels in autism. Pharmacopsychiatry. 2012;45:241–243. doi: 10.1055/s-0032-1301914. [DOI] [PubMed] [Google Scholar]
- Essa MM, Guillemin GJ, Waly MI, Al-Sharbati MM, Al-Farsi YM, Hakkim FL, et al. Increased markers of oxidative stress in autistic children of the Sultanate of Oman. Biol Trace Elem Res. 2012;147:25–27. doi: 10.1007/s12011-011-9280-x. [DOI] [PubMed] [Google Scholar]
- Lakshmi Priya MD, Geetha A. A biochemical study on the level of proteins and their percentage of nitration in the hair and nail of autistic children. Clinica Chimica Acta; Int J Clin Chem. 2011;412:1036–1042. doi: 10.1016/j.cca.2011.02.021. [DOI] [PubMed] [Google Scholar]
- Melnyk S, Fuchs GJ, Schulz E, Lopez M, Kahler SG, Fussell JJ, et al. Metabolic imbalance associated with methylation dysregulation and oxidative damage in children with autism. J Autism Dev Disorders. 2012;42:367–377. doi: 10.1007/s10803-011-1260-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James SJ, Melnyk S, Jernigan S, Cleves MA, Halsted CH, Wong DH, et al. Metabolic endophenotype and related genotypes are associated with oxidative stress in children with autism. Am J Med Genet B Neuropsychiatr Genet. 2006;141B:947–956. doi: 10.1002/ajmg.b.30366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose S, Melnyk S, Pavliv O, Bai S, Nick TG, Frye RE, et al. Evidence of oxidative damage and inflammation associated with low glutathione redox status in the autism brain. Translational Psychiatry. 2012;2:e134. doi: 10.1038/tp.2012.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajdel-Sulkowska EM, Xu M, McGinnis W, Koibuchi N. Brain region-specific changes in oxidative stress and neurotrophin levels in autism spectrum disorders (ASD) Cerebellum. 2011;10:43–48. doi: 10.1007/s12311-010-0223-4. [DOI] [PubMed] [Google Scholar]
- Sweeten TL, Posey DJ, Shankar S, McDougle CJ. High nitric oxide production in autistic disorder: a possible role for interferon-gamma. Biol Psychiatry. 2004;55:434–437. doi: 10.1016/j.biopsych.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Laranjinha J, Santos RM, Lourenco CF, Ledo A, Barbosa RM. Nitric oxide signaling in the brain: translation of dynamics into respiration control and neurovascular coupling. Ann N Y Acad Sci. 2012;1259:10–18. doi: 10.1111/j.1749-6632.2012.06582.x. [DOI] [PubMed] [Google Scholar]
- Brown GC, Neher JJ. Inflammatory neurodegeneration and mechanisms of microglial killing of neurons. Mol Neurobiol. 2010;41:242–247. doi: 10.1007/s12035-010-8105-9. [DOI] [PubMed] [Google Scholar]
- Sarti P, Arese M, Forte E, Giuffre A, Mastronicola D. Mitochondria and nitric oxide: chemistry and pathophysiology. Adv Exp Med Biol. 2012;942:75–92. doi: 10.1007/978-94-007-2869-1_4. [DOI] [PubMed] [Google Scholar]
- James SJ, Rose S, Melnyk S, Jernigan S, Blossom S, Pavliv O, et al. Cellular and mitochondrial glutathione redox imbalance in lymphoblastoid cells derived from children with autism. FASEB J. 2009;23:2374–2383. doi: 10.1096/fj.08-128926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaiman C, Huffman L, Elliott GR.Sapropterin as a treatment for Autism Spectrum Disorders: a double-blind, placebo-controlled trial J Child Adol Psychop 2013(in press). [DOI] [PubMed]