Abstract
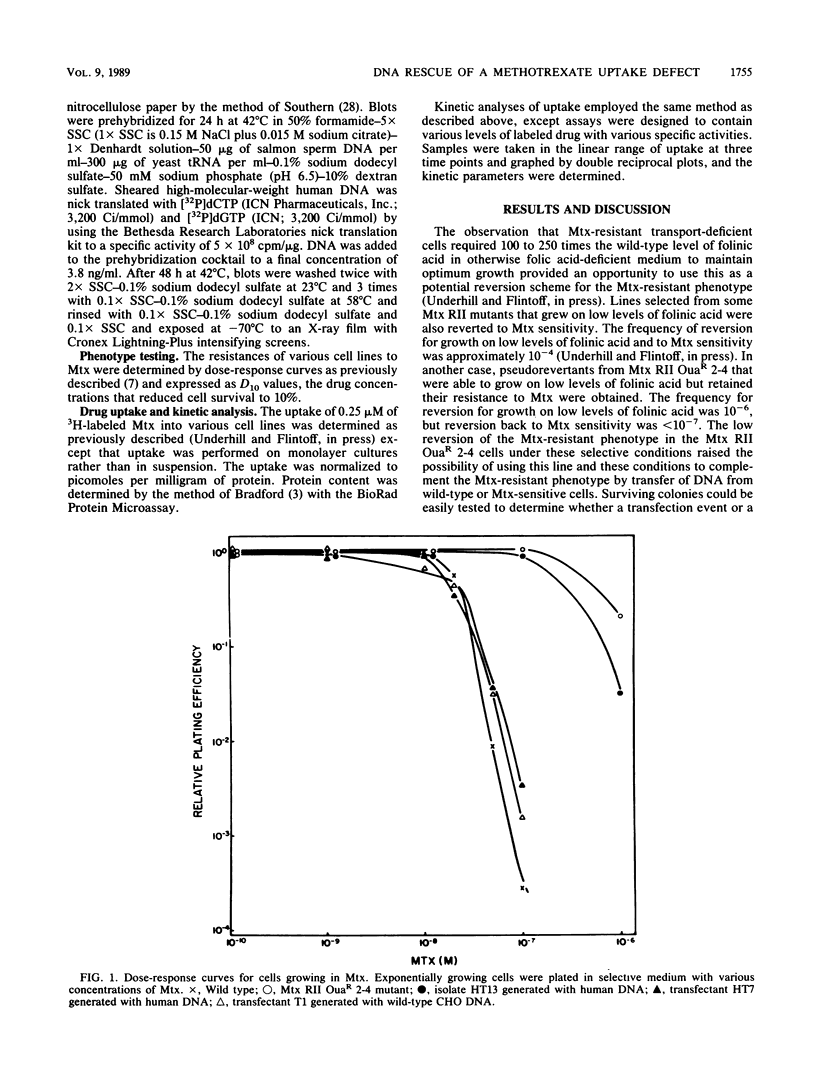
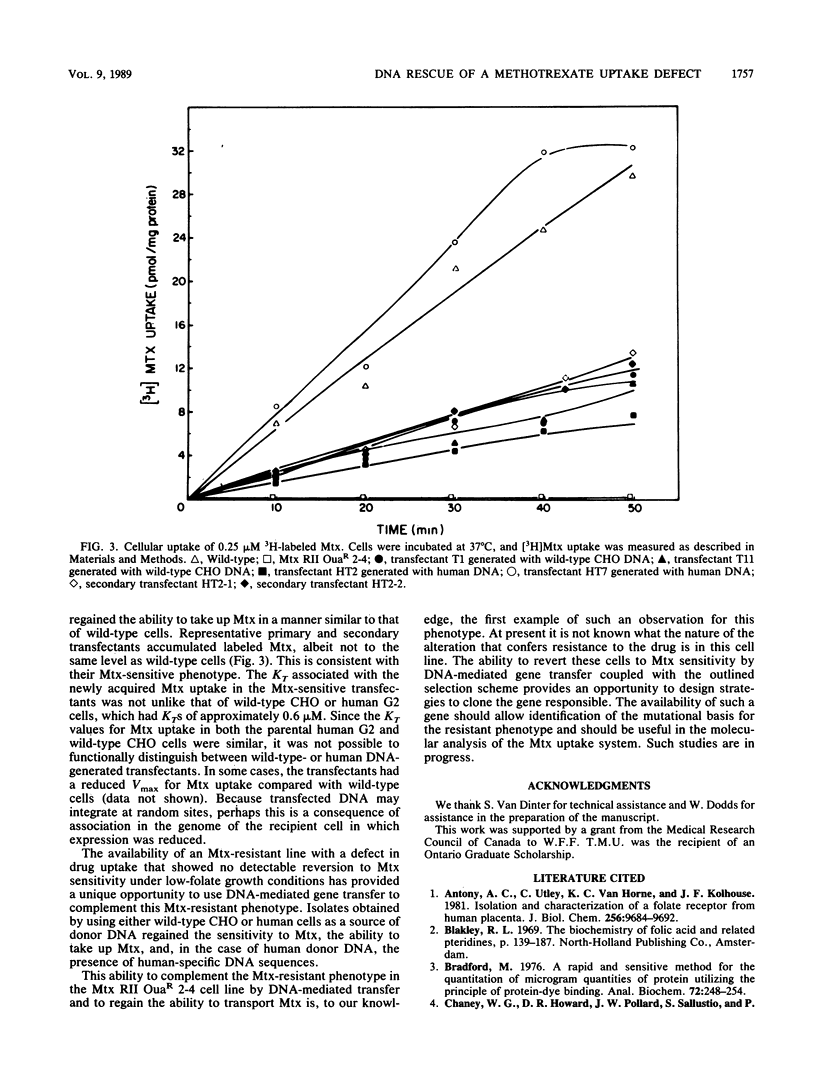
A methotrexate-resistant Chinese hamster ovary cell line deficient in methotrexate uptake has been complemented to methotrexate sensitivity by transfection with DNA isolated from either wild-type Chinese hamster ovary or human G2 cells. Primary and secondary transfectants regained the ability to take up methotrexate in a manner similar to that of wild-type cells, and in the case of those transfected with human DNA, to contain human-specific DNA sequences. The complementation by DNA-mediated gene transfer of this methotrexate-resistant phenotype provides a basis for the cloning of a gene involved in methotrexate uptake.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antony A. C., Utley C., Van Horne K. C., Kolhouse J. F. Isolation and characterization of a folate receptor from human placenta. J Biol Chem. 1981 Sep 25;256(18):9684–9692. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Chaney W. G., Howard D. R., Pollard J. W., Sallustio S., Stanley P. High-frequency transfection of CHO cells using polybrene. Somat Cell Mol Genet. 1986 May;12(3):237–244. doi: 10.1007/BF01570782. [DOI] [PubMed] [Google Scholar]
- Elwood P. C., Kane M. A., Portillo R. M., Kolhouse J. F. The isolation, characterization, and comparison of the membrane-associated and soluble folate-binding proteins from human KB cells. J Biol Chem. 1986 Nov 25;261(33):15416–15423. [PubMed] [Google Scholar]
- Flintoff W. F., Davidson S. V., Siminovitch L. Isolation and partial characterization of three methotrexate-resistant phenotypes from Chinese hamster ovary cells. Somatic Cell Genet. 1976 May;2(3):245–261. doi: 10.1007/BF01538963. [DOI] [PubMed] [Google Scholar]
- Flintoff W. F., Nagainis C. R. Transport of methotrexate in Chinese hamster ovary cells: a mutant defective in methotrexate uptake and cell binding. Arch Biochem Biophys. 1983 Jun;223(2):433–440. doi: 10.1016/0003-9861(83)90607-0. [DOI] [PubMed] [Google Scholar]
- Flintoff W. F., Spindler S. M., Siminovitch L. Genetic characterization of methotrexate-resistant chinese hamster ovary cells. In Vitro. 1976 Nov;12(11):749–757. doi: 10.1007/BF02835450. [DOI] [PubMed] [Google Scholar]
- Flintoff W., Saya L. The selection of wild-type revertants from methotrexate permeability mutants. Somatic Cell Genet. 1978 Mar;4(2):143–156. doi: 10.1007/BF01538980. [DOI] [PubMed] [Google Scholar]
- Gross-Bellard M., Oudet P., Chambon P. Isolation of high-molecular-weight DNA from mammalian cells. Eur J Biochem. 1973 Jul 2;36(1):32–38. doi: 10.1111/j.1432-1033.1973.tb02881.x. [DOI] [PubMed] [Google Scholar]
- Henderson G. B., Montague-Wilkie B. Irreversible inhibitors of methotrexate transport in L1210 cells. Characteristics of inhibition by an N-hydroxysuccinimide ester of methotrexate. Biochim Biophys Acta. 1983 Oct 26;735(1):123–130. doi: 10.1016/0005-2736(83)90267-5. [DOI] [PubMed] [Google Scholar]
- Henderson G. B., Suresh M. R., Vitols K. S., Huennekens F. M. Transport of folate compounds in L1210 cells: kinetic evidence that folate influx proceeds via the high-affinity transport system for 5-methyltetrahydrofolate and methotrexate. Cancer Res. 1986 Apr;46(4 Pt 1):1639–1643. [PubMed] [Google Scholar]
- Henderson G. B., Zevely E. M. Affinity labeling of the 5-methyltetrahydrofolate/methotrexate transport protein of L1210 cells by treatment with an N-hydroxysuccinimide ester of [3H]methotrexate. J Biol Chem. 1984 Apr 10;259(7):4558–4562. [PubMed] [Google Scholar]
- Henderson G. B., Zevely E. M. Structural requirements for anion substrates of the methotrexate transport system in L1210 cells. Arch Biochem Biophys. 1983 Mar;221(2):438–446. doi: 10.1016/0003-9861(83)90162-5. [DOI] [PubMed] [Google Scholar]
- Kane M. A., Portillo R. M., Elwood P. C., Antony A. C., Kolhouse J. F. The influence of extracellular folate concentration on methotrexate uptake by human KB cells. Partial characterization of a membrane-associated methotrexate binding protein. J Biol Chem. 1986 Jan 5;261(1):44–49. [PubMed] [Google Scholar]
- Kano Y., Ohnuma T., Holland J. F. Folate requirements of methotrexate-resistant human acute lymphoblastic leukemia cell lines. Blood. 1986 Aug;68(2):586–591. [PubMed] [Google Scholar]
- Lowy I., Pellicer A., Jackson J. F., Sim G. K., Silverstein S., Axel R. Isolation of transforming DNA: cloning the hamster aprt gene. Cell. 1980 Dec;22(3):817–823. doi: 10.1016/0092-8674(80)90558-9. [DOI] [PubMed] [Google Scholar]
- Luhrs C. A., Pitiranggon P., da Costa M., Rothenberg S. P., Slomiany B. L., Brink L., Tous G. I., Stein S. Purified membrane and soluble folate binding proteins from cultured KB cells have similar amino acid compositions and molecular weights but differ in fatty acid acylation. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6546–6549. doi: 10.1073/pnas.84.18.6546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick J. I., Susten S. S., Rader J. I., Freisheim J. H. Studies of a methotrexate binding protein fraction from L1210 lymphocyte plasma membranes. Eur J Cancer. 1979 Nov;15(11):1377–1386. doi: 10.1016/0014-2964(79)90115-4. [DOI] [PubMed] [Google Scholar]
- Podskalny J. M., Takeda S., Silverman R. E., Tran D., Carpentier J. L., Orci L., Gorden P. Insulin receptors and bioresponses in a human liver cell line (Hep G-2). Eur J Biochem. 1985 Jul 15;150(2):401–407. doi: 10.1111/j.1432-1033.1985.tb09034.x. [DOI] [PubMed] [Google Scholar]
- Sadasivan E., da Costa M., Rothenberg S. P., Brink L. Purification, properties, and immunological characterization of folate-binding proteins from human leukemia cells. Biochim Biophys Acta. 1987 Jul 16;925(1):36–47. doi: 10.1016/0304-4165(87)90145-0. [DOI] [PubMed] [Google Scholar]
- Sekiguchi T., Yoshida M. C., Sekiguchi M., Nishimoto T. Isolation of a human X chromosome-linked gene essential for progression from G1 to S phase of the cell cycle. Exp Cell Res. 1987 Apr;169(2):395–407. doi: 10.1016/0014-4827(87)90200-x. [DOI] [PubMed] [Google Scholar]
- Shaham M., Adler B., Ganguly S., Chaganti R. S. Transfection of normal human and Chinese hamster DNA corrects diepoxybutane-induced chromosomal hypersensitivity of Fanconi anemia fibroblasts. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5853–5857. doi: 10.1073/pnas.84.16.5853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirotnak F. M., Goutas L. J., Jacobsen D. M., Mines L. S., Barrueco J. R., Gaumont Y., Kisliuk R. L. Carrier-mediated transport of folate compounds in L1210 cells. Initial rate kinetics and extent of duality of entry routes for folic acid and diastereomers of 5-methyltetrahydrohomofolate in the presence of physiological anions. Biochem Pharmacol. 1987 May 15;36(10):1659–1667. doi: 10.1016/0006-2952(87)90051-7. [DOI] [PubMed] [Google Scholar]
- Sirotnak F. M., Goutas L. J., Mines L. S. Extent of the requirement for folate transport by L1210 cells for growth and leukemogenesis in vivo. Cancer Res. 1985 Oct;45(10):4732–4734. [PubMed] [Google Scholar]
- Sirotnak F. M. Obligate genetic expression in tumor cells of a fetal membrane property mediating "folate" transport: biological significance and implications for improved therapy of human cancer. Cancer Res. 1985 Sep;45(9):3992–4000. [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Weber C. A., Salazar E. P., Stewart S. A., Thompson L. H. Molecular cloning and biological characterization of a human gene, ERCC2, that corrects the nucleotide excision repair defect in CHO UV5 cells. Mol Cell Biol. 1988 Mar;8(3):1137–1146. doi: 10.1128/mcb.8.3.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerveld A., Hoeijmakers J. H., van Duin M., de Wit J., Odijk H., Pastink A., Wood R. D., Bootsma D. Molecular cloning of a human DNA repair gene. Nature. 1984 Aug 2;310(5976):425–429. doi: 10.1038/310425a0. [DOI] [PubMed] [Google Scholar]
- Yang C. H., Dembo M., Sirotnak F. M. Relationships between carrier-mediated transport of folate compounds by L1210 leukemia cells: evidence for multiplicity of entry routes with different kinetic properties expressed in plasma membrane vesicles. J Membr Biol. 1983;75(1):11–20. doi: 10.1007/BF01870795. [DOI] [PubMed] [Google Scholar]
- Yang C. H., Sirotnak F. M., Mines L. S. Further studies on a novel class of genetic variants of the L1210 cell with increased folate analogue transport inward. Transport properties of a new variant, evidence for increased levels of a specific transport protein, and its partial characterization following affinity labeling. J Biol Chem. 1988 Jul 15;263(20):9703–9709. [PubMed] [Google Scholar]
- da Costa M., Rothenberg S. P. Characterization of the folate-binding proteins associated with the plasma membrane of rat liver. Biochim Biophys Acta. 1988 Apr 22;939(3):533–541. doi: 10.1016/0005-2736(88)90100-9. [DOI] [PubMed] [Google Scholar]