Table 1.
Tandem diene diboration/double allylation of dicarbonyls.a
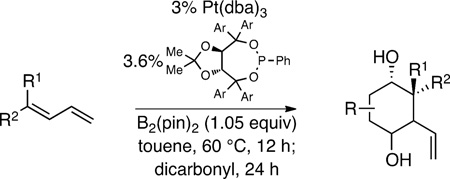 | |||||
|---|---|---|---|---|---|
| entry | R1 | R2 | dicarbonyl | product | d.r. e.r. yield |
| 1 | prenyl | Me | 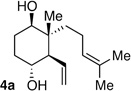 |
5:1 dr 6:4 er 76 % |
|
| 2 | Me | prenyl |  |
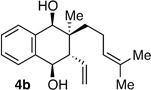 |
2.8:1 dr 96:4 er 83% |
| 3 | prenyl | Me | 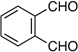 |
 |
1.2:1 dr 97:3 er 72% |
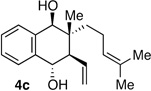
| 4b | Me | CH2OSiR3 | 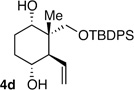 |
10:1 dr 97:3 er 71% |
|
| 5 | pentyl | H | 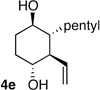 |
>20:1 dr 91:9 er 39% |
|
| 6 | -(CH2)5- | 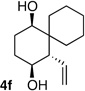 |
9:1 dr 88:12 er 72% |
||
| 7 | Me | prenyl | 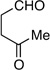 |
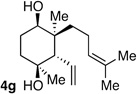 |
11:1 dr 97:3 er 77% |
| 8 | Me | prenyl | 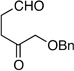 |
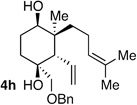 |
>20:1 dr 97:3 er 60% |
| 9 | Me | prenyl | 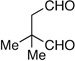 |
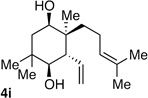 |
>20:1 dr 97:3 er 54% |
Entries 1, 4, and 6 employed 2 equivalents of dicarbonyl; entries 2, 3, 7, and 8 employed 1 equiv. dicarbonyl; entry 5 employed 3 equiv dicarbonyl; entry 9 employed 2 equivalents of diene/B2(pin)2. Diastereoselectivity determined by 1H NMR analysis; yield refers to isolated yield of the regioisomer mixture.
(S,S) ligand employed for this experiment.
