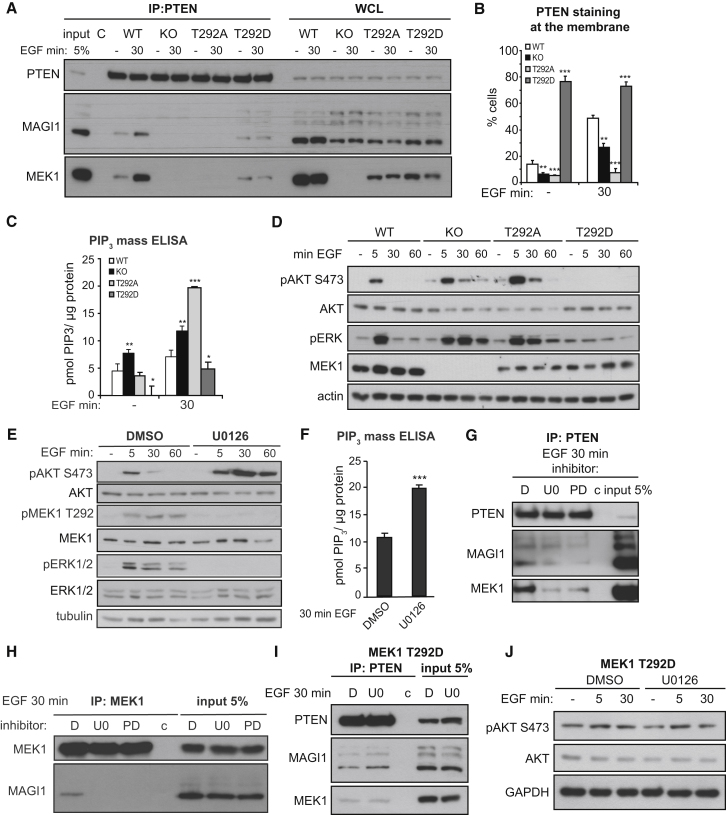
Figure 6.
T292 Phosphorylation, but Not MEK1 Kinase Activity, Promotes MEK1/MAGI1/PTEN Binding and Decreases PIP3 Pathway Activation
(A) WT, KO, and MEK1 mutant fibroblasts were stimulated with EGF. PTEN, MAGI1, and MEK1 in endogenous PTEN IPs and WCL were detected by immunoblotting.
(B–D) MEK1 WT, KO, and mutant fibroblasts were stimulated with EGF. PTEN membrane recruitment was determined by immunofluorescence. The plot in (B) depicts the percentage of cells showing membrane PTEN. The plot in (C) shows PIP3 levels, determined by ELISA. pAKT, pERK, AKT, MEK, and tubulin (loading control) were detected by immunoblotting (D).
(E and F) WT MEFs were pretreated with MEK inhibitors or DMSO and stimulated with EGF. Phosphorylation and expression of AKT, ERK, MEK1, and tubulin (loading control) were determined by immunoblotting (E). Intracellular PIP3 levels in WT MEFs pretreated with U0126 or DMSO and stimulated with EGF were measured by ELISA (F).
(G and H) WT fibroblasts pretreated with DMSO or MEK inhibitors (U0126, PD0325901) and stimulated with EGF were subjected to PTEN (G) or MEK1 IP (H). PTEN, MAGI1, and MEK1 were detected by immunoblotting. c, nonspecific binding to the beads.
(I and J) T292D MEK1 mutant MEFs were treated with U0126 or DMSO and stimulated with EGF. PTEN, MEK1, and MAGI1 in PTEN IPs (I) and pAKT, AKT, and GAPDH (loading control) in WCL (J) were visualized by immunoblotting. In (J), c represents nonspecific binding to the beads.
The values in (B), (C), and (F) show the mean of three experiments ±SD. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001. See also Figure S5.