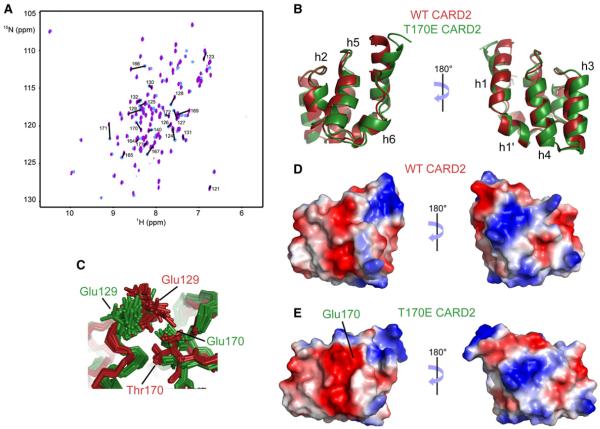
Figure 4. Comparison of NMR Spectra and Structures of Wild-type CARD2 and the T170E CARD2 Mutant.
(A) Changes of peak positions is shown on the two-dimensional HSQC spectra of the wild-type and T170E mutant proteins. Assignments are shown for residues with a chemical shift perturbation above an arbitrary threshold (see supporting information).
(B) Lowest-energy structures of the wild-type (red) and T170E mutant (green) CARD2.
(C–E) Focus on the conformations of Glu129 and Thr170/Glu170 sidechains (C). Surface electrostatics of WT CARD2 (D) and T170E CARD2 (E) are shown in the same orientation as in (B).
See full structural details and dynamics in Figure S3.