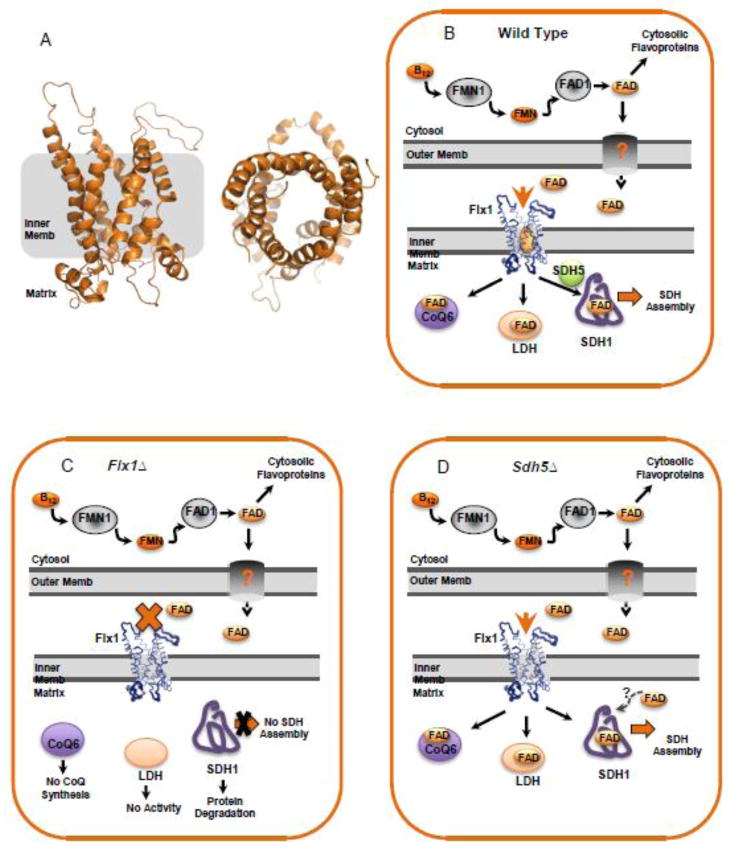
Fig 2.
The putative role of Flx1 in the trafficking of FAD in the mitochondria and its effect on Sdh1 flavinylation. (A) Model of Flx1 structure based on template PDB 2LCK, an integral membrane protein in the mitochondrial anion carrier protein family [47] showing a side view (left) and a top-down view (right). (B) A theoretical model of FAD trafficking based primarily on the proposed import role of Flx1 by Tzagoloff [40]. An alternative model has been put forth by the Barile group that proposes Flx1 as an exporter of FAD [42]. In the wild-type mitochondria of S. cerevisiae FAD is synthesized from riboflavin from the sequential actions of FMN1 and FAD1 in the cytosol. A certain portion of the FAD enters the mitochondria through an unknown outer membrane transporter and into the mitochondrial matrix through the carrier Flx1. The matrix FAD is utilized for the enzymes including CoQ6, LDH, and Sdh1. (C) In the absence of Flx1, FAD levels in the mitochondrial matrix are thought to decrease and the activities of CoQ6 and LDH are dramatically impaired. Moreover, SDH does not assemble presumably due to the lack of a Sdh1-FAD interaction leading to apo Sdh1 instability. flx1Δ cells do not render the IM completely impermeable; over expression of FAD1 partly complements the CoQ6 and LDH defect. (D) In the absence of Sdh5, SDH assembly still proceeds, presumably due to the fact that FAD can still bind to Sdh1.