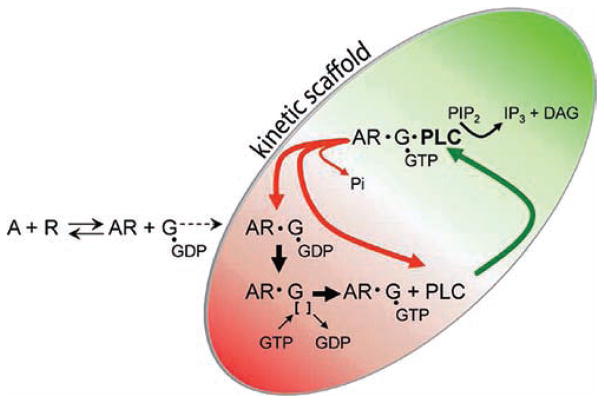
Figure 3.
Kinetic model of GPCR-promoted Gαq-dependent activation of PLC-β. Agonists (A) interact reversibly with GPCRs (R) to promote coupling to GDP-bound heterotrimeric Gq (G) and consequential dissociation of GDP and binding of GTP to Gαq. GTP—Gαq then binds with high affinity to PLC-β to activate the lipase and promote a high rate of conversion of PtdIns(4,5)P2 (PIP2) to Ins(1,4,5)P3 (IP3) and diacylglycerol. Bound PLC-β markedly enhances the rate of GTP hydrolysis by Gαq, resulting in a return of the G protein to the inactive GDP-bound state and dissociation of PLC-β. In the continued presence of agonist, activated R stays bound to Gq through multiple cycles of GTP binding and hydrolysis since the rate of dissociation of R from GTP-bound Gαq is much slower than that of the PLC-β- promoted return of the G protein to the GDP-bound state. This phenomenon whereby agonist-activated R is retained in a signaling complex with the G protein through multiple cycles of GTP binding and hydrolysis has been referred to by Ross,96, Neubig and co-workers, 122 and others as a “kinetic scaffold”.