Abstract
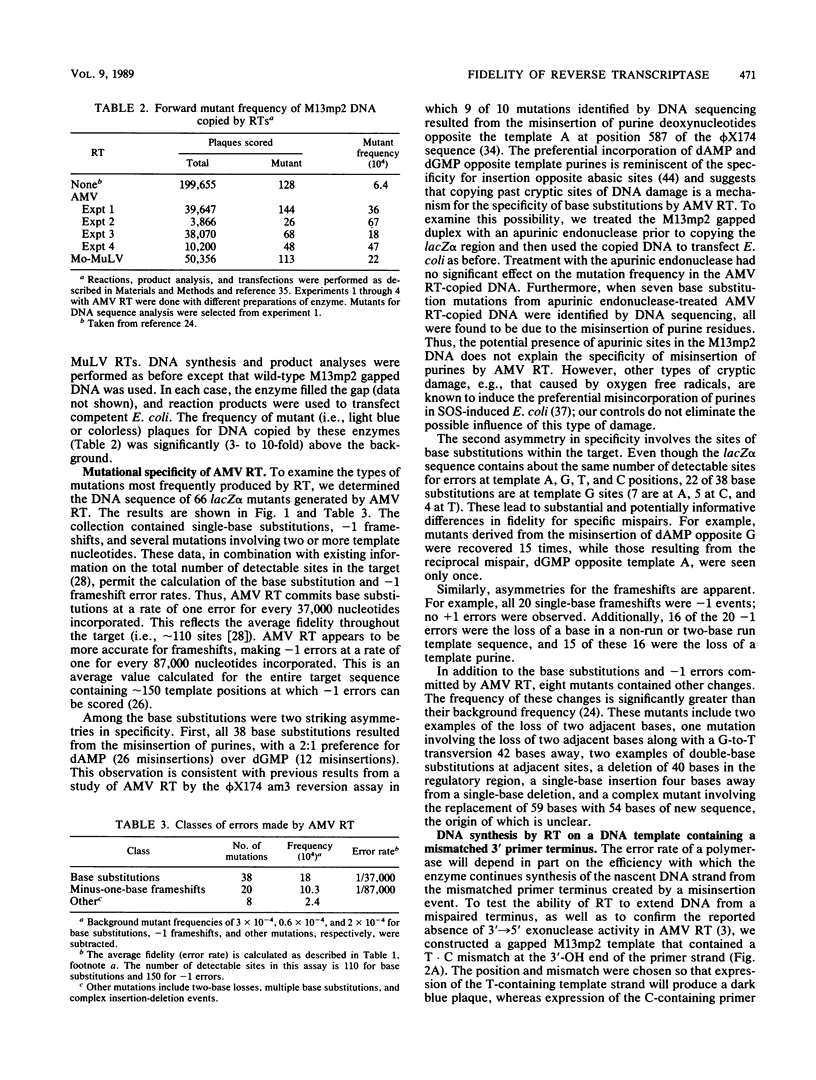
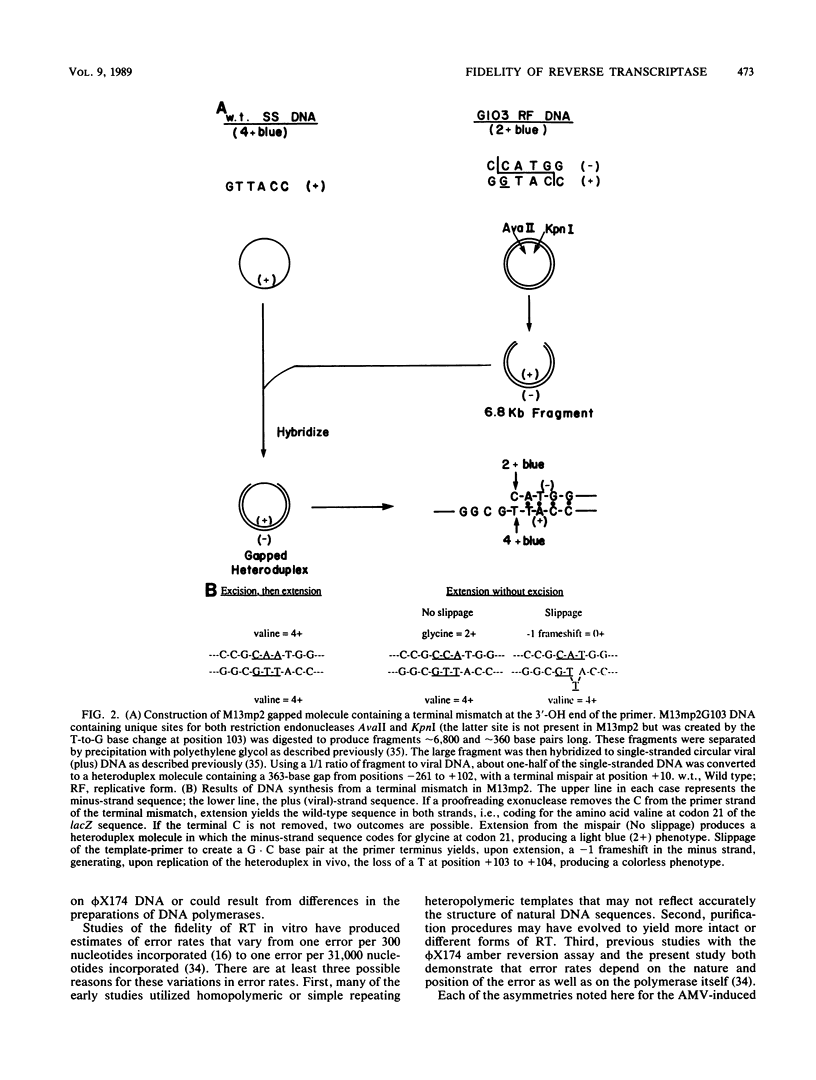
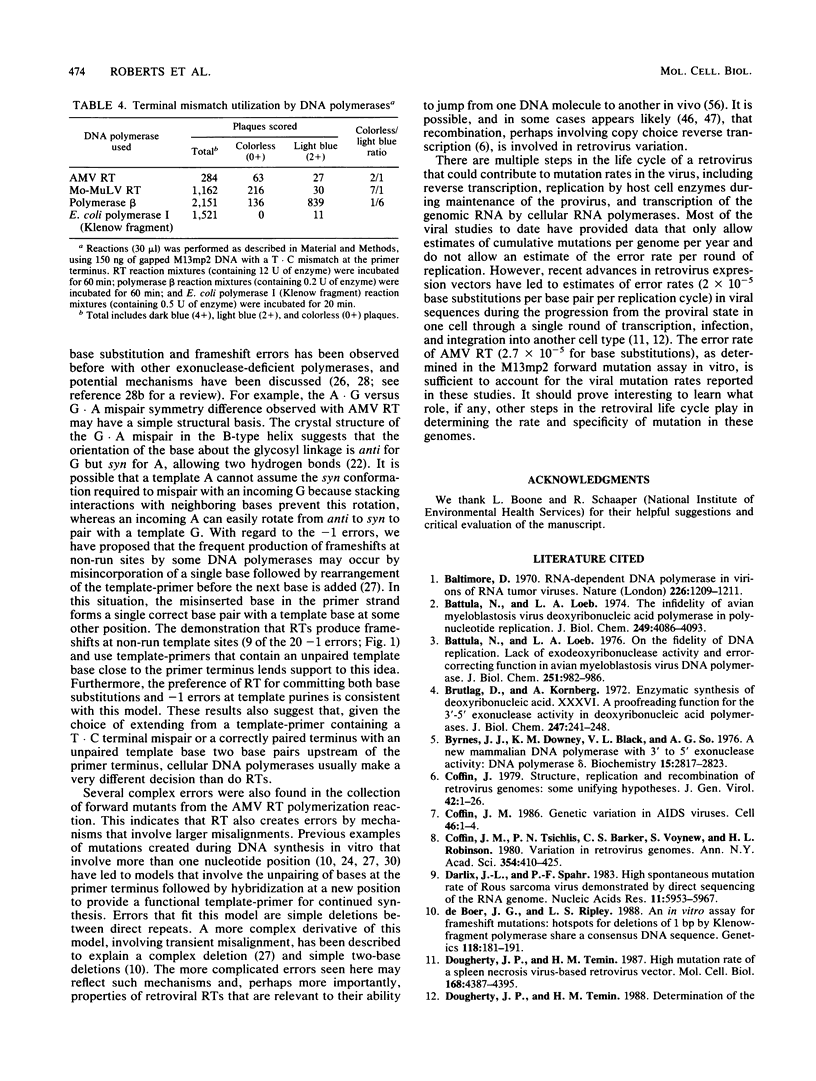
We determined the fidelity of avian myeloblastosis virus and Moloney murine leukemia virus reverse transcriptases (RTs) during DNA synthesis in vitro using the M13mp2 lacZ alpha gene as a mutational target. Both RTs commit an error approximately once for every 30,000 nucleotides polymerized. DNA sequence analysis of mutants generated in a forward mutation assay capable of detecting many types of errors demonstrated that avian myeloblastosis virus RT produced a variety of different mutations. The majority (58%) were single-base substitutions; all of which resulted from the misincorporation of either dAMP or dGMP. Minus-one frameshifts were also common, composing about 30% of the mutations. In addition to single-base events, eight mutants contained sequence changes involving from 2 to 59 bases. The frequency of these mutants suggests that, at least during DNA synthesis in vitro, RTs also commit errors by mechanisms other than classical base miscoding and misalignment. We examined the ability of RTs to synthesize DNA from a mismatched primer terminus at a sequence where the mismatched base was complementary to the next base in the template. Unlike cellular DNA polymerases which polymerize from the mismatched template-primer, RTs preferred to polymerize from a rearranged template-primer containing a matched terminal base pair and an unpaired base in the template strand. The unusual preference for this substrate suggests that the interactions between RTs and the template-primer are different from those of cellular DNA polymerases. The overall error rate of RT in vitro is sufficient to account for the estimated mutation rate of these viruses.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baltimore D. RNA-dependent DNA polymerase in virions of RNA tumour viruses. Nature. 1970 Jun 27;226(5252):1209–1211. doi: 10.1038/2261209a0. [DOI] [PubMed] [Google Scholar]
- Battula N., Loeb L. A. On the fidelity of DNA replication. Lack of exodeoxyribonuclease activity and error-correcting function in avian myeloblastosis virus DNA polymerase. J Biol Chem. 1976 Feb 25;251(4):982–986. [PubMed] [Google Scholar]
- Battula N., Loeb L. A. The infidelity of avian myeloblastosis virus deoxyribonucleic acid polymerase in polynucleotide replication. J Biol Chem. 1974 Jul 10;249(13):4086–4093. [PubMed] [Google Scholar]
- Brutlag D., Kornberg A. Enzymatic synthesis of deoxyribonucleic acid. 36. A proofreading function for the 3' leads to 5' exonuclease activity in deoxyribonucleic acid polymerases. J Biol Chem. 1972 Jan 10;247(1):241–248. [PubMed] [Google Scholar]
- Byrnes J. J., Downey K. M., Black V. L., So A. G. A new mammalian DNA polymerase with 3' to 5' exonuclease activity: DNA polymerase delta. Biochemistry. 1976 Jun 29;15(13):2817–2823. doi: 10.1021/bi00658a018. [DOI] [PubMed] [Google Scholar]
- Coffin J. M. Genetic variation in AIDS viruses. Cell. 1986 Jul 4;46(1):1–4. doi: 10.1016/0092-8674(86)90851-2. [DOI] [PubMed] [Google Scholar]
- Coffin J. M. Structure, replication, and recombination of retrovirus genomes: some unifying hypotheses. J Gen Virol. 1979 Jan;42(1):1–26. doi: 10.1099/0022-1317-42-1-1. [DOI] [PubMed] [Google Scholar]
- Coffin J. M., Tsichlis P. N., Barker C. S., Voynow S., Robinson H. L. Variation in avian retrovirus genomes. Ann N Y Acad Sci. 1980;354:410–425. doi: 10.1111/j.1749-6632.1980.tb27982.x. [DOI] [PubMed] [Google Scholar]
- Darlix J. L., Spahr P. F. High spontaneous mutation rate of Rous sarcoma virus demonstrated by direct sequencing of the RNA genome. Nucleic Acids Res. 1983 Sep 10;11(17):5953–5967. doi: 10.1093/nar/11.17.5953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty J. P., Temin H. M. Determination of the rate of base-pair substitution and insertion mutations in retrovirus replication. J Virol. 1988 Aug;62(8):2817–2822. doi: 10.1128/jvi.62.8.2817-2822.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duyk G., Longiaru M., Cobrinik D., Kowal R., deHaseth P., Skalka A. M., Leis J. Circles with two tandem long terminal repeats are specifically cleaved by pol gene-associated endonuclease from avian sarcoma and leukosis viruses: nucleotide sequences required for site-specific cleavage. J Virol. 1985 Nov;56(2):589–599. doi: 10.1128/jvi.56.2.589-599.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopinathan K. P., Weymouth L. A., Kunkel T. A., Loeb L. A. Mutagenesis in vitro by DNA polymerase from an RNA tumour virus. Nature. 1979 Apr 26;278(5707):857–859. doi: 10.1038/278857a0. [DOI] [PubMed] [Google Scholar]
- Hahn B. H., Shaw G. M., Taylor M. E., Redfield R. R., Markham P. D., Salahuddin S. Z., Wong-Staal F., Gallo R. C., Parks E. S., Parks W. P. Genetic variation in HTLV-III/LAV over time in patients with AIDS or at risk for AIDS. Science. 1986 Jun 20;232(4757):1548–1553. doi: 10.1126/science.3012778. [DOI] [PubMed] [Google Scholar]
- Hizi A., Joklik W. K. RNA-dependent DNA polymerase of avian sarcoma virus B77. I. Isolation and partial characterization of the alpha, beta2, and alphabeta forms of the enzyme. J Biol Chem. 1977 Apr 10;252(7):2281–2289. [PubMed] [Google Scholar]
- Huang W. M., Lehman I. R. On the exonuclease activity of phage T4 deoxyribonucleic acid polymerase. J Biol Chem. 1972 May 25;247(10):3139–3146. [PubMed] [Google Scholar]
- Kaguni L. S., DiFrancesco R. A., Lehman I. R. The DNA polymerase-primase from drosophila melanogaster embryos. Rate and fidelity of polymerization on single-stranded DNA templates. J Biol Chem. 1984 Jul 25;259(14):9314–9319. [PubMed] [Google Scholar]
- Kane C. M., Linn S. Purification and characterization of an apurinic/apyrimidinic endonuclease from HeLa cells. J Biol Chem. 1981 Apr 10;256(7):3405–3414. [PubMed] [Google Scholar]
- Kunkel T. A., Alexander P. S. The base substitution fidelity of eucaryotic DNA polymerases. Mispairing frequencies, site preferences, insertion preferences, and base substitution by dislocation. J Biol Chem. 1986 Jan 5;261(1):160–166. [PubMed] [Google Scholar]
- Kunkel T. A., Bebenek K. Recent studies of the fidelity of DNA synthesis. Biochim Biophys Acta. 1988 Nov 10;951(1):1–15. doi: 10.1016/0167-4781(88)90020-6. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A., Eckstein F., Mildvan A. S., Koplitz R. M., Loeb L. A. Deoxynucleoside [1-thio]triphosphates prevent proofreading during in vitro DNA synthesis. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6734–6738. doi: 10.1073/pnas.78.11.6734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A., Gopinathan K. P., Dube D. K., Snow E. T., Loeb L. A. Rearrangements of DNA mediated by terminal transferase. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1867–1871. doi: 10.1073/pnas.83.6.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A., Loeb L. A. Fidelity of mammalian DNA polymerases. Science. 1981 Aug 14;213(4509):765–767. doi: 10.1126/science.6454965. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A. Mutational specificity of depurination. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1494–1498. doi: 10.1073/pnas.81.5.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A., Sabatino R. D., Bambara R. A. Exonucleolytic proofreading by calf thymus DNA polymerase delta. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4865–4869. doi: 10.1073/pnas.84.14.4865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A., Schaaper R. M., Beckman R. A., Loeb L. A. On the fidelity of DNA replication. Effect of the next nucleotide on proofreading. J Biol Chem. 1981 Oct 10;256(19):9883–9889. [PubMed] [Google Scholar]
- Kunkel T. A., Schaaper R. M., Loeb L. A. Depurination-induced infidelity of deoxyribonucleic acid synthesis with purified deoxyribonucleic acid replication proteins in vitro. Biochemistry. 1983 May 10;22(10):2378–2384. doi: 10.1021/bi00279a012. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A., Soni A. Exonucleolytic proofreading enhances the fidelity of DNA synthesis by chick embryo DNA polymerase-gamma. J Biol Chem. 1988 Mar 25;263(9):4450–4459. [PubMed] [Google Scholar]
- Kunkel T. A. The mutational specificity of DNA polymerase-beta during in vitro DNA synthesis. Production of frameshift, base substitution, and deletion mutations. J Biol Chem. 1985 May 10;260(9):5787–5796. [PubMed] [Google Scholar]
- Livingston D. M., Richardson C. C. Deoxyribonucleic acid polymerase III of Escherichia coli. Characterization of associated exonuclease activities. J Biol Chem. 1975 Jan 25;250(2):470–478. [PubMed] [Google Scholar]
- Loeb L. A., James E. A., Waltersdorph A. M., Klebanoff S. J. Mutagenesis by the autoxidation of iron with isolated DNA. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3918–3922. doi: 10.1073/pnas.85.11.3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb L. A., Kunkel T. A. Fidelity of DNA synthesis. Annu Rev Biochem. 1982;51:429–457. doi: 10.1146/annurev.bi.51.070182.002241. [DOI] [PubMed] [Google Scholar]
- Mizutani S., Temin H. M. Incorporation of noncomplementary nucleotides at high frequencies by ribodeoxyvirus DNA polymerases and Escherichia coli DNA polymerase I. Biochemistry. 1976 Apr 6;15(7):1510–1516. doi: 10.1021/bi00652a023. [DOI] [PubMed] [Google Scholar]
- O'Rear J. J., Temin H. M. Spontaneous changes in nucleotide sequence in proviruses of spleen necrosis virus, an avian retrovirus. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1230–1234. doi: 10.1073/pnas.79.4.1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyland M. E., Loeb L. A. On the fidelity of DNA replication. Isolation of high fidelity DNA polymerase-primase complexes by immunoaffinity chromatography. J Biol Chem. 1987 Aug 5;262(22):10824–10830. [PubMed] [Google Scholar]
- Roberts J. D., Kunkel T. A. Fidelity of a human cell DNA replication complex. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7064–7068. doi: 10.1073/pnas.85.19.7064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaaper R. M., Kunkel T. A., Loeb L. A. Infidelity of DNA synthesis associated with bypass of apurinic sites. Proc Natl Acad Sci U S A. 1983 Jan;80(2):487–491. doi: 10.1073/pnas.80.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff R. D., Grandgenett D. P. Virus-coded origin of a 32,000-dalton protein from avian retrovirus cores: structural relatedness of p32 and the beta polypeptide of the avian retrovirus DNA polymerase. J Virol. 1978 Oct;28(1):279–291. doi: 10.1128/jvi.28.1.279-291.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields A., Witte W. N., Rothenberg E., Baltimore D. High frequency of aberrant expression of Moloney murine leukemia virus in clonal infections. Cell. 1978 Jul;14(3):601–609. doi: 10.1016/0092-8674(78)90245-3. [DOI] [PubMed] [Google Scholar]
- Shimotohno K., Temin H. M. Spontaneous variation and synthesis in the U3 region of the long terminal repeat of an avian retrovirus. J Virol. 1982 Jan;41(1):163–171. doi: 10.1128/jvi.41.1.163-171.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirover M. A., Loeb L. A. Infidelity of DNA synthesis: a general property of RNA tumor viruses. Biochem Biophys Res Commun. 1974 Nov 27;61(2):410–414. doi: 10.1016/0006-291x(74)90972-3. [DOI] [PubMed] [Google Scholar]
- Sirover M. A., Loeb L. A. On the fidelity of DNA replication. Effect of metal activators during synthesis with avian myeloblastosis virus DNA polymerase. J Biol Chem. 1977 Jun 10;252(11):3605–3610. [PubMed] [Google Scholar]
- Smith D. B., Inglis S. C. The mutation rate and variability of eukaryotic viruses: an analytical review. J Gen Virol. 1987 Nov;68(Pt 11):2729–2740. doi: 10.1099/0022-1317-68-11-2729. [DOI] [PubMed] [Google Scholar]
- Springgate C. F., Battula N., Loeb L. A. Infidelity of DNA synthesis by reverse transcriptase. Biochem Biophys Res Commun. 1973 May 15;52(2):401–406. doi: 10.1016/0006-291x(73)90725-0. [DOI] [PubMed] [Google Scholar]
- TEMIN H. M. Mixed infection with two types of Rous sarcoma virus. Virology. 1961 Feb;13:158–163. doi: 10.1016/0042-6822(61)90049-6. [DOI] [PubMed] [Google Scholar]
- Tanese N., Goff S. P. Domain structure of the Moloney murine leukemia virus reverse transcriptase: mutational analysis and separate expression of the DNA polymerase and RNase H activities. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1777–1781. doi: 10.1073/pnas.85.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varmus H. Retroviruses. Science. 1988 Jun 10;240(4858):1427–1435. doi: 10.1126/science.3287617. [DOI] [PubMed] [Google Scholar]
- Verma I. M. Studies on reverse transcriptase of RNA tumor viruses III. Properties of purified Moloney murine leukemia virus DNA polymerase and associated RNase H. J Virol. 1975 Apr;15(4):843–854. doi: 10.1128/jvi.15.4.843-854.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weymouth L. A., Loeb L. A. Infidelity of DNA synthesis by themperature-sensitive DNA polymerases from RNA tumor viruses. Biochim Biophys Acta. 1977 Oct 4;478(3):305–315. doi: 10.1016/0005-2787(77)90148-4. [DOI] [PubMed] [Google Scholar]
- de Boer J. G., Ripley L. S. An in vitro assay for frameshift mutations: hotspots for deletions of 1 bp by Klenow-fragment polymerase share a consensus DNA sequence. Genetics. 1988 Feb;118(2):181–191. doi: 10.1093/genetics/118.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]


