Figure 2.
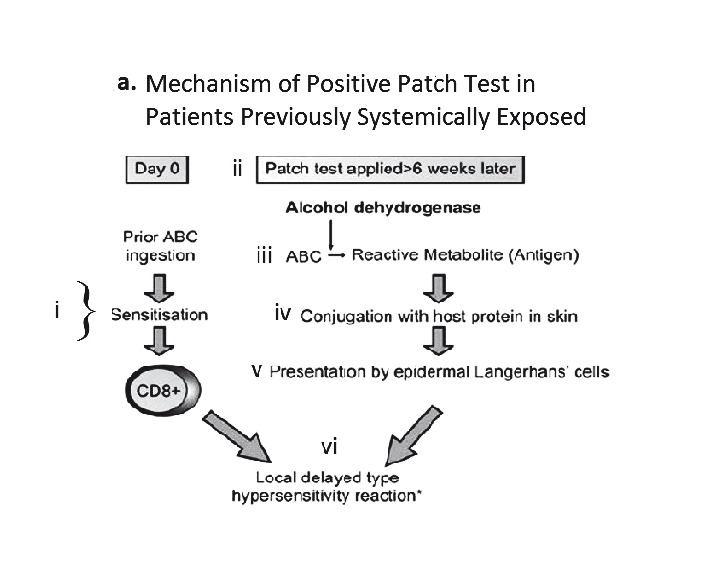
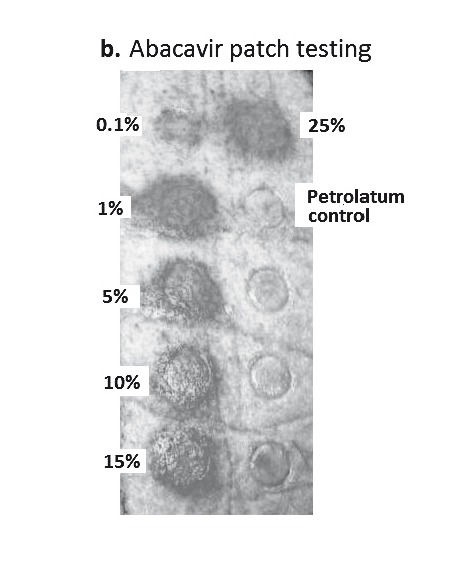
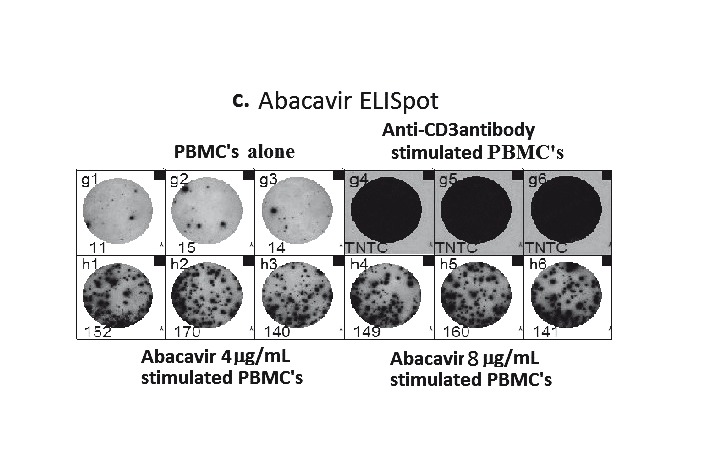
A. Proposed mechanism of positive patch test in patients previously systemically exposed. (i) After sensitisation and immunological priming, (ii) the patch test requires abacavir from the patch to penetrate into the skin (epidermal layer). (iii) Abacavir is then metabolised into a reactive metabolite, by the enzyme alcohol dehydrogenase (ADH). (iv) The metabolite then conjugates with host proteins to form an antigen. (v) Once recognised by MHC expressing, antigen-presenting epidermal Langerhans cells (LCs), they are processed and presented to effector cells of the immune system. (vi)This in turn triggers peptide-specific CD8+ T-cells to migrate into the skin, proliferate, and release proinflammatory cytokines and chemokines causing a localised response. B. Abacavir patch test with positive patch tests in an HLA-B*5701 positive patient 2 months following abacavir HSS. Documented with increasing concentrations from 0.1% to 25% w/w (abacavir/petrolatum) and negative petrolatum control (below the 25% abacavir test patch square). C. ABC-induced IFN-γ responses by ELISpot assay (shown as spot forming units (SFU)/200,000). Clockwise from top left - Incubation of PBMCs from an HLA-B*5701 positive patient 72 months following abacavir HSS. In 4 and 8 µg/mL of abacavir (pharmacologically relevant concentrations) produces INF-γ responses in the form of (blue) spots; PBMCs only, no INF-γ responses were detected; and lastly the addition of anti-CD3 antibodies to PBMCs acts as a positive control.
