Abstract
The molecular study of the erect wing (ewg) locus was initiated by isolating DNA in the 1A8-1B1 interval of the X chromosome. Previous developmental genetic analyses of the mutant alleles at the ewg locus have demonstrated that the wild-type ewg product is essential during embryogenesis and is required postembryonically at least for the development of the indirect flight muscle. To define the ewg-encoding DNA, chromosomal breakpoints that genetically flank the ewg locus were used. P-element-mediated transformation followed by subsequent rescue of the ewg-lethal alleles has defined a 11.5-kilobase genomic fragment as encoding the ewg locus. Northern blot analysis of transcription from this DNA has revealed a complex pattern of transcription with respect to both size and developmental profile. Tissue distribution of putative ewg transcription was examined by in situ hybridization to 6- to 14-h-old embryonic sections. These sections revealed that the expression of putative ewg messages is limited to the central nervous system-derived structures and not observed within the mesoderm during this developmental stage.
Full text
PDF

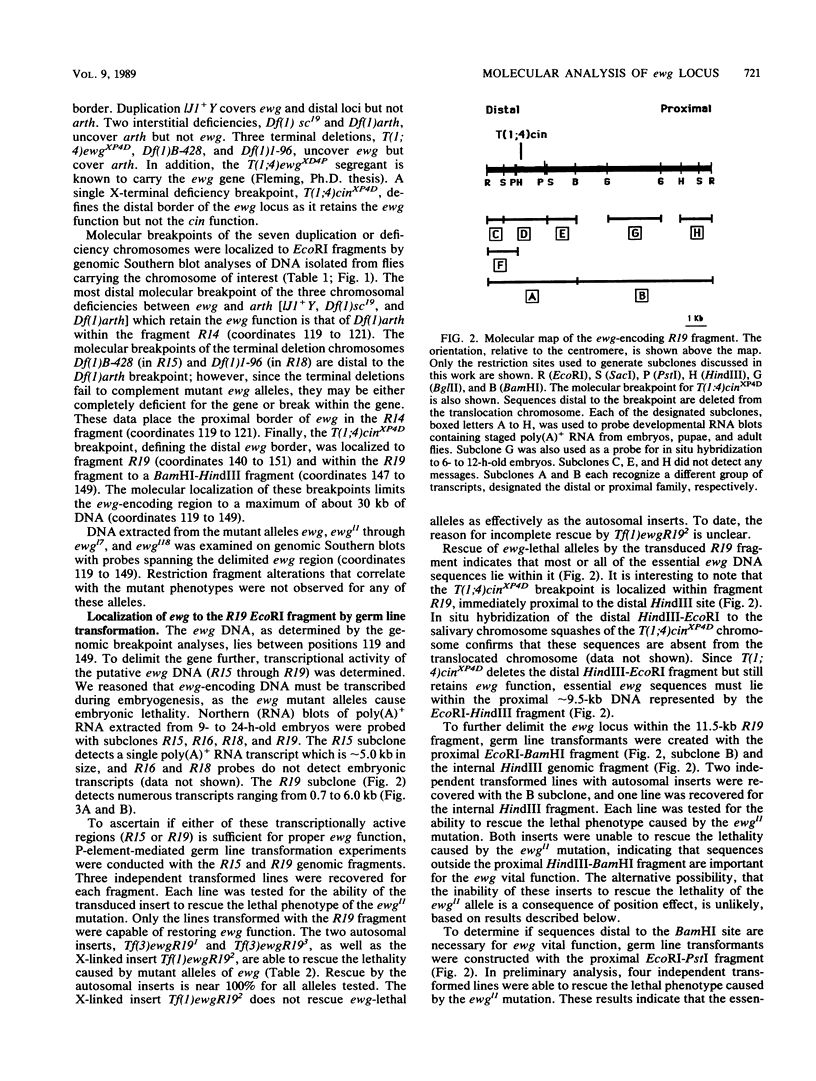
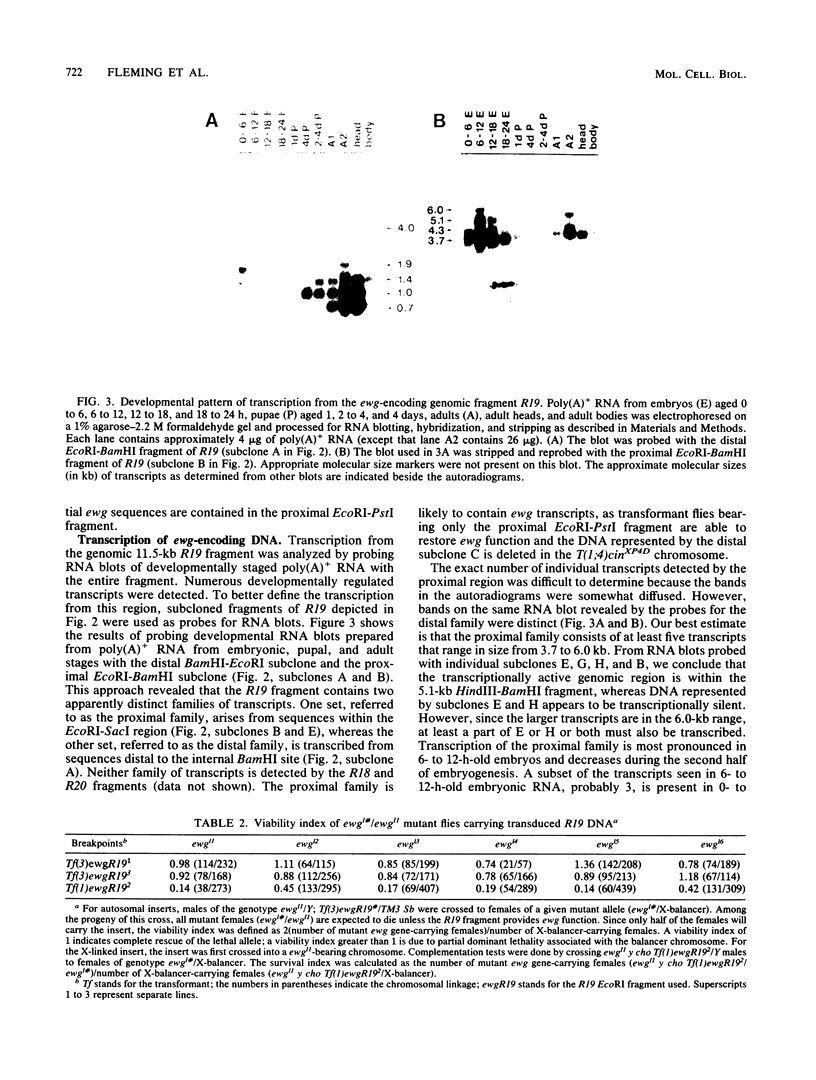


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernstein S. I., Mogami K., Donady J. J., Emerson C. P., Jr Drosophila muscle myosin heavy chain encoded by a single gene in a cluster of muscle mutations. 1983 Mar 31-Apr 6Nature. 302(5907):393–397. doi: 10.1038/302393a0. [DOI] [PubMed] [Google Scholar]
- Biessmann H. Molecular analysis of the yellow gene (y) region of Drosophila melanogaster. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7369–7373. doi: 10.1073/pnas.82.21.7369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner P. H., Adams T. R. Neural induction of chick myoblast differentiation in culture. Dev Biol. 1982 Mar;90(1):175–184. doi: 10.1016/0012-1606(82)90223-8. [DOI] [PubMed] [Google Scholar]
- Campos A. R., Rosen D. R., Robinow S. N., White K. Molecular analysis of the locus elav in Drosophila melanogaster: a gene whose embryonic expression is neural specific. EMBO J. 1987 Feb;6(2):425–431. doi: 10.1002/j.1460-2075.1987.tb04772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campuzano S., Carramolino L., Cabrera C. V., Ruíz-Gómez M., Villares R., Boronat A., Modolell J. Molecular genetics of the achaete-scute gene complex of D. melanogaster. Cell. 1985 Feb;40(2):327–338. doi: 10.1016/0092-8674(85)90147-3. [DOI] [PubMed] [Google Scholar]
- Deak I. I. Mutations of Drosophila melanogaster that affect muscles. J Embryol Exp Morphol. 1977 Aug;40:35–63. [PubMed] [Google Scholar]
- Deak I. I. Thoracic duplications in the mutant wingless of Drosophila and their effect on muscles and nerves. Dev Biol. 1978 Oct;66(2):422–441. doi: 10.1016/0012-1606(78)90249-x. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Govind C. K., Kent K. S. Transformation of fast fibres to slow prevented by lack of activity in developing lobster muscle. Nature. 1982 Aug 19;298(5876):755–757. doi: 10.1038/298755a0. [DOI] [PubMed] [Google Scholar]
- Hall J. C. Genetics of the nervous system in Drosophila. Q Rev Biophys. 1982 May;15(2):223–479. doi: 10.1017/s0033583500004844. [DOI] [PubMed] [Google Scholar]
- Harris A. J. Embryonic growth and innervation of rat skeletal muscles. I. Neural regulation of muscle fibre numbers. Philos Trans R Soc Lond B Biol Sci. 1981 Jul 16;293(1065):257–277. doi: 10.1098/rstb.1981.0076. [DOI] [PubMed] [Google Scholar]
- Karlik C. C., Coutu M. D., Fyrberg E. A. A nonsense mutation within the act88F actin gene disrupts myofibril formation in Drosophila indirect flight muscles. Cell. 1984 Oct;38(3):711–719. doi: 10.1016/0092-8674(84)90266-6. [DOI] [PubMed] [Google Scholar]
- Karlik C. C., Fyrberg E. A. An insertion within a variably spliced Drosophila tropomyosin gene blocks accumulation of only one encoded isoform. Cell. 1985 May;41(1):57–66. doi: 10.1016/0092-8674(85)90061-3. [DOI] [PubMed] [Google Scholar]
- Koana T., Hotta Y. Isolation and characterization of flightless mutants in Drosophila melanogaster. J Embryol Exp Morphol. 1978 Jun;45:123–143. [PubMed] [Google Scholar]
- Lawrence P. A., Johnston P. The muscle pattern of a segment of Drosophila may be determined by neurons and not by contributing myoblasts. Cell. 1986 May 23;45(4):505–513. doi: 10.1016/0092-8674(86)90282-5. [DOI] [PubMed] [Google Scholar]
- Lefevre G. The distribution of randomly recovered X-ray-induced sex-linked genetic effects in Drosophila melanogaster. Genetics. 1981 Nov-Dec;99(3-4):461–480. doi: 10.1093/genetics/99.3-4.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahaffey J. W., Coutu M. D., Fyrberg E. A., Inwood W. The flightless Drosophila mutant raised has two distinct genetic lesions affecting accumulation of myofibrillar proteins in flight muscles. Cell. 1985 Jan;40(1):101–110. doi: 10.1016/0092-8674(85)90313-7. [DOI] [PubMed] [Google Scholar]
- McLennan I. S. Neural dependence and independence of myotube production in chicken hindlimb muscles. Dev Biol. 1983 Aug;98(2):287–294. doi: 10.1016/0012-1606(83)90359-7. [DOI] [PubMed] [Google Scholar]
- Mogami K., Hotta Y. Isolation of Drosophila flightless mutants which affect myofibrillar proteins of indirect flight muscle. Mol Gen Genet. 1981;183(3):409–417. doi: 10.1007/BF00268758. [DOI] [PubMed] [Google Scholar]
- Pirrotta V., Hadfield C., Pretorius G. H. Microdissection and cloning of the white locus and the 3B1-3C2 region of the Drosophila X chromosome. EMBO J. 1983;2(6):927–934. doi: 10.1002/j.1460-2075.1983.tb01523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirrotta V., Steller H., Bozzetti M. P. Multiple upstream regulatory elements control the expression of the Drosophila white gene. EMBO J. 1985 Dec 16;4(13A):3501–3508. doi: 10.1002/j.1460-2075.1985.tb04109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell J. A., Friedman B., Cossi A. Tissue culture study of murine muscular dysgenesis: role of spontaneous action potential generation in the regulation of muscle maturation. Ann N Y Acad Sci. 1979;317:550–570. doi: 10.1111/j.1749-6632.1979.tb56576.x. [DOI] [PubMed] [Google Scholar]
- Robinow S., White K. The locus elav of Drosophila melanogaster is expressed in neurons at all developmental stages. Dev Biol. 1988 Apr;126(2):294–303. doi: 10.1016/0012-1606(88)90139-x. [DOI] [PubMed] [Google Scholar]
- Rubin G. M., Spradling A. C. Genetic transformation of Drosophila with transposable element vectors. Science. 1982 Oct 22;218(4570):348–353. doi: 10.1126/science.6289436. [DOI] [PubMed] [Google Scholar]
- Simon J. A., Sutton C. A., Lobell R. B., Glaser R. L., Lis J. T. Determinants of heat shock-induced chromosome puffing. Cell. 1985 Apr;40(4):805–817. doi: 10.1016/0092-8674(85)90340-x. [DOI] [PubMed] [Google Scholar]
- Spradling A. C., Rubin G. M. Transposition of cloned P elements into Drosophila germ line chromosomes. Science. 1982 Oct 22;218(4570):341–347. doi: 10.1126/science.6289435. [DOI] [PubMed] [Google Scholar]
- Zehring W. A., Wheeler D. A., Reddy P., Konopka R. J., Kyriacou C. P., Rosbash M., Hall J. C. P-element transformation with period locus DNA restores rhythmicity to mutant, arrhythmic Drosophila melanogaster. Cell. 1984 Dec;39(2 Pt 1):369–376. doi: 10.1016/0092-8674(84)90015-1. [DOI] [PubMed] [Google Scholar]